Abstract
Objective
The goal of the present study was to localize and quantify immunoreactive protaglandin H synthase 1 (PGHS-1), PGHS-2, and Fos expression by immunohistochemistry in the fetal brain 30 minutes and 2 hours after the onset of a 10-minute period of cerebral hypoperfusion in barodenervated and chemodenervated fetal sheep.
Methods
Fetal sheep of known gestational age were studied intact or sinoaortic denervated. Fetuses were sacrificed and tissues recovered for immunohistochemistry or real-time polymerase chain reaction measurements of protein and mRNA, respectively. Some fetuses were subjected to brachiocephalic occlusion, produced by inflation of an extravascular balloon occluder around the brachiocephalic artery. Immunohistochemistry results were quantified using image analysis, and mRNA was quantified by estimation of cycle threshold in generation of PGHS-1 or PGHS-2 amplicons.
Results
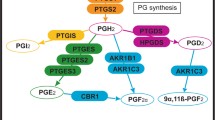
Sinoaortic denervation by itself did not alter the abundance of PGHS-1 or PGHS-2 protein in any brain region, although the denervation did reduce the abundance of PGHS-1 mRNA in hypothalamus. We assessed PGHS-1, PGHS-2, and Fos immunoreactive protein abundance by image analysis of histologic sections stained for the respective proteins using immunohistochemistry. Cerebral hypoperfusion increased the intensity of staining of immunoreactive PGHS-1, PGHS-2, and Fos in the anterior pituitary, hippocampus, and cerebellum. In the cerebral microvasculature, the intensity of PGHS-1 and Fos was significantly greater, and in the cerebral cortex, the intensity of PGHS-2 was significantly greater. Changes in the amount of immunostaining in the nucleus of tractus solitarius and paraventricular nucleus were not statistically significant.
Conclusion
Cerebral hypoperfusion altered the expression and distribution of prostaglandin biosynthetic enzymes in ovine fetal brain by a mechanism that is independent of baroreceptor and chemoreceptor afferent activity.
Similar content being viewed by others
References
Wang LH, Hajibeigi A, Xu XM, Loose-Mitchell D, Wu K. Characterization of the promotor of human prostaglandin H synthase-1 gene. Biochem Biophys Res Commun 1993;190:406–11.
Xie W, Merrill JR, Bradshaw WS, Simmons DL. Structural determination and promotor analysis of the chicken mitogen-inducible prostaglandin G/H synthase gene and genetic mapping of the murine homolog. Arch Biochem Biophys 1993;300:247–52.
Sairanen T, Ristimaki A, Karalainen-Lindsberg ML, Paetau A, Kaste M, Lindsberg PJ. Cyclooxygenase-2 is induced globally in infarcted human brain. Ann Neurol 1998;43:738–47.
Degi R, Bari F, Thrikawala N, et al. Effects of anoxic stress on prostaglandin H synthase isoforms in piglet brain. Brain Res Dev Brain Res 1998;107:265–76.
Chiu R, Boyle WJ, Meek J, Smeal T, Hunter T, Karin M. The c-fos protein interacts with c-jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell 1988;54:541–52.
Kindy MS, Carney JP, Dempsey RJ, Carney JM. Ischemic induction of protooncogene expression in gerbil brain. J Mol Neurosci 1991;2:217–28.
Onodera H, Kogure K, Ono Y, Igarashi K, Kiyota Y, Nagaoka A. Proto-oncogene c-fos is transiently induced in the rat cerebral cortex after forebrain ischemia. Neurosci Lett 1989;98:101–4.
Shimazu M, Mizushima H, Sasaki K, et al. Expression of c-fos in the rat cerebral cortex after focal ischemia and reperfusion. Brain Res Bull 1994;33:689–97.
Breder CD, Smith WL, Raz A, et al. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J Comp Neurol 1992;322:409–38.
Breder CD, DeWitt DL, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 1995;355:296–315.
Lee SH, Soyoola E, Chanmugam P, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem 1992;267:25934–8.
O’Bamon MK, Miller JC, Chang JW, Kaplan MD, Coleman PD. Interleukin-1 beta induces prostaglandin G/H synthase-2 (cyclooxygenase-2) in primary murine astrocyte cultures. J Neurochem 1996;66:2532–40.
Habib A, Creminon C, Frobert Y, Grassi J, Pradelles P, Mclouf J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of cyclooxygenase-2. J Biol Chem 1993;268:23448–54.
Holtz ML, Kindy MS, Craddock S, Moore RW, Pettigrew LC. Induction of PGH synthase and c-fos mRNA during early reperfusion of ischemic rat brain. Mol Brain Res 1996;35:339–43.
Tong H, Richards EM, Wood CE. Prostaglandin endoperoxide synthase-2 abundance is increased in brain tissues of late-gestation fetal sheep in response to cerebral hypoperfusion. J Soc Gynecol Investig 1999;6:127–35.
Collaco-Morales Y, Aspey B, Harrison M, deBelleroche J. Cyclooxygenase-2 messenger RNA induction in focal cerebral ischemia. J Cereb Blood Flow Metab 1996;16:1366–72.
Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in late-gestation fetal sheep. Am J Physiol 1998;275:R735–41.
Bradford MM. A refined and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
Zar JH. Biostatistical analysis. Englewood Cliffs, New Jersey: Prentice-Hall, 1984.
Tong H, Dhillon H, Wood CE. Induction of PGHS-2 mRNA in response to cerebral hypoperfusion in late-gestation fetal sheep. Prostaglandins Other Lipid Mediators 2000;62:165–72.
Norton JL, Adamson SL, Bocking AD, Han VK. Prostaglandin-H synthase-1 (PGHS-1) gene is expressed in specific neurons of the brain of the late gestation ovine fetus. Brain Res Dev Brain Res 1996;95:79–96.
Nedergaard M, Diemer NH. Focal ischemia of the rat brain, with special reference to the influence of plasma glucose concentration. Acta Neuropathol 1987;73:131–7.
Koistinaho J, Hokfelt T. Altered gene expression in brain ischemia. Neuro Rep 1997;8:i–viii.
Chemtob S, Beharry K, Rex J, Varma DR, Aranda JV. Changes in cerebrovascular prostaglandins and thromboxane as a function of systemic blood pressure. Circ Res 1990;67:674–82.
Tong H, Wood CE. Indomethacin attenuates the cerebral blood flow response to hypotension in late-gestation fetal sheep. Am J Physiol 1999;277:R1268–73.
Tong H, Richards E, Wood CE. Prostaglandin endoperoxide synthase-2 abundance is increased in brain tissues of late-gestation fetal sheep in response to cerebral hypoperfusion. J Soc Gynecol Investig 1999;6:127–35.
Domoki F, Veltkamp R, Thrikawala N, et al. Ischemia-reperfusion rapidly increases COX-2 expression in piglet cerebral arteries. Am J Physiol 1999;277:H1207–14.
Ikeda J, Nakajima T, Gerfen C, Nowak TS. In situ hybridizatoin analysis of c-fos and prodynorphin mRNA levels and distribution in gerbil hippocampus after transient ischemia. Stroke 1990;21(Suppl):1–16.
Ceccatelli S, Villar MJ, Goldstein M, Hokfelt T. Expression of c-fos immunoreactivity in transmitter-characterized neurons after stress. Proc Natl Acad Sci U S A 1989;86:9569–73.
Hoffman GE, McDonald T, Shedwick R, Nathanielsz PW. Activation of cFos in ovine fetal corticotropin-releasing hormone neurons at the time of parturition. Endocrinology 1991;129:3227–33.
Kononen J, Honkaniemi J, Alho H, Koistinaho J, Iadorola M, Pelto-Huikko M. Fos-like immunoreactivity in the rat hypothalamic-pituitary-axis after immobilization stress. Endocrinology 1992;130:3041–7.
Gubits RM, Burke RE, Casey-Mcintosh G, Bandele A, Munell F. Immediate early gene induction after neonatal hypoxia-ischemia. Mol Brain Res 1993;18:228–38.
Caubert J. C-fos proto-oncogene expression in the nervous system during mouse development. Mol Cell Biol 1989;9:2269–72.
Ruppert C, Wille W. Proto-oncogene c-fos is highly inducible by disruption of neonatal but not mature brain tissue. Mol Brain Res 1987;2:51–6.
Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in lategestation fetal sheep. Am J Physiol 1998;275:R735–41.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grant HD33053 from National Institute of Child Health and Human Development and a Grant-in-Aid from the Florida/Puerto Rico Affiliate of the American Heart Association to Dr. Wood, a predoctoral fellowship from the Florida/ Puerto Rico Affiliate of the American Heart Association to Dr. Tong, and a postdoctoral NRSA from the National Institute of Child Health and Human Development to Dr. Gridley.
Rights and permissions
About this article
Cite this article
Tong, H., Gridley, K.E. & Wood, C.E. Induction of Immunoreactive Prostaglandin H Synthases 1 and 2 and Fos in Response to Cerebral Hypoperfusion in Late-Gestation Fetal Sheep. Reprod. Sci. 9, 342–350 (2002). https://doi.org/10.1177/107155760200900604
Published:
Issue Date:
DOI: https://doi.org/10.1177/107155760200900604