Abstract
Objective
Using neuropeptide and enzyme markers to autonomic nerves, we sought to demonstrate and quantify the nerve types contained within the uterosacral ligaments (USLs) and cardinal ligaments (CLs) that are divided during radical hysterectomy (RH).
Methods
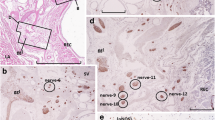
Cross-sectional biopsies were collected from the lateral third of the USL and the CL in 24 women who had an RH for cervical cancer, and from the uterine insertion of these ligaments in 11 women who had a simple hysterectomy for benign disease. We applied indirect immunofluorescence with FITC-conjugated secondary antibodies, using polyclonal primary antibodies to neuropeptide markers that predominate within somatic and autonomic nerves, to show different populations of the following nerve types within the biopsies: neuropeptide Y (NPY) and tyrosine hydroxylase (TH) for sympathetic nerves; vasoactive intestinal polypeptide (VIP) for parasympathetic nerves; substance P (SP) for nociceptive and sensory-motor nerves; and calcitonin gene-related peptide (CGRP) for sensory and sensory-motor nerves. The percentage area of immunoreactivity (PAI), determined by a computer-assisted image analyzed attached to a fluorescent microscope, was used as an objective quantitative measure of nerve density. Confocal microscopy was used to determine the composition and spatial arrangement of nerve fibers in the ligaments.
Results
The PAI was greater for all markers tested in both the USL and CL (P < .001) in RH compared with simple hysterectomy biopsies. For RH specimens, the PAI was greater for the sympathetic, sensory, and sensory-motor nerve markers in the USL compared with the CL (P < .01), but the PAI for VIP was similar (P > .05). Conversely, excluding the large trunks and associated ganglia, the free nerve fiber PAI in the CL was greater than that of the USL for all nerve markers (P < .001). The staining of peripheral autonomic ganglia and associated fibers, for NPY and TH, indicates that some sympathetic nerves are preganglionic with their cell bodies within the pelvic plexus.
Conclusions
Significantly more autonomic nerves are transected in the more lateral division of the uterine supporting ligaments during a radical hysterectomy than during a simple hysterectomy. Sympathetic, parasympathetic, sensory, and sensory-motor nerve types are present within the CL and USL. The proportions of each nerve type differ between the two ligaments, and sympathetic nerves in the USL are the single largest nerve type. The uterine supporting ligaments are a major pathway for autonomic nerves to the pelvic organs.
Similar content being viewed by others
References
Seski JC, Diokno AC. Bladder dysfunction after radical abdominal hysterectomy. Am J Obstet Gynecol 1977;128:643–51.
Forney P. The effects of radical hysterectomy on bladder physiology. Am J Obstet Gynecol 1980;138:374–82.
Zanolla R, Monzeglio C, Campo B, Ordesi G, Balzarini A, Martino G. Bladder and urethral dysfunction after radical abdominal hysterectomy. J Surg Oncol 1985;28:190–4.
Barnes W, Waggoner S, Delgado G, et al. Manometric characterization of rectal dysfunction following radical hysterectomy. Gynecol Oncol 1991;42:116–9.
Flay LD, Mathews JHL. The effects of radiotherapy and surgery on the sexual function of women treated for cervical cancer. Int J Radiat Oncol Biol Phys 1995;31:399–404.
Andersen BL. Sexual functioning morbidity among cancer survivors, current status and future research directions. Cancer 1985;55:1835–42.
Andersen BL. Predicting sexual and psychologic morbidity and improving the quality of life for women with gynecologic cancer. Cancer 1993;71(4 suppl):1678–90.
Andersen BL. Surviving cancer. Cancer 1994;74:1484–95.
Butler-Manuel SA, Summerville K, Ford AM, et al. Self-assessment of morbidity following radical hysterectomy for cervical cancer. J Obstet Gynaecol 1999;19:180–3.
Baljet B, Drukker J. The extrinsic innervation of the pelvic organs in the female rat. Acta Anat 1980;107:241–67.
Gu J, Polak JM, Su HC, Blank MA, Morrison JFB, Bloom SR. Demonstration of paracervical ganglion origin for the vasoactive intestinal polypeptide-containing nerves of the rat uterus using retrograde tracing techniques combined with immunocytochem-istry and denervation procedures. Neurosci Lett 1984;51:377–82.
Inyama CO, Hacker GW, Gu J, Dhal D, Bloom SR, Polak JM. Cytochemical relationships in the paracervical ganglion (Fran-kenhauser) of rat studied by immunocytochemistry. Neurosci Lett 1985;55:311–5.
Lundberg LM, Alm P, Wharton J, Polak JM. Protein gene product 9.5 (PGP 9.5). A new neuronal marker visualizing the whole uterine innervation and pregnancy-induced and developmental changes in the guinea pig. Histochem Cell Biol 1988;90:9–17.
Mitchell BS. Morphology and neurochemistry of the pelvic and paracervical ganglia. Histol Histopathol 1993;8:761–73.
Papka RE, Thompson BD, Schmidt HHW. Identification of uterine-related sympathetic neurons in the rat inferior mesenteric ganglion: Neurotransmitter content and afferent input. J Auton Nerv Syst 1996;59:51–9.
Dhami D, Mitchell BS. The effects of decentralisation on substance P-immunoreactivity in the anterior major pelvic ganglion of the male guinea pig. J Auton Nerv Syst 1992;38:167–76.
Lakomy M, Kaleczyc W, Majewski M, Mienkiewicz W. Peptidergic innervation of the bovine vagina and uterus. Acta Histochem 1995;97:53–66.
Papka RE, McNeil DL, Thompson D, Schmidt HHW. Nitric oxide nerves in the uterus are parasympathetic, sensory, and contain neuropeptides. Cell Tissue Res 1995;279:339–49.
Mundy AR. An anatomical explanation for bladder dysfunction following rectal and uterine surgery. Br J Urol 1982;54:501–4.
Twombly GH, Landers D. The innervation of the bladder with reference to radical hysterectomy. Am J Obstet Gynecol 1956;71:1291.
Smith PH, Ballantyne B. The neuro anatomical basis of the urinary bladder dysfunction following major pelvic surgery. Br J Surg 1968;55:929–33.
Hockel M, Konerding MA, Heussel CP. Liposuction-assisted nerve-sparing extended radical hysterectomy: Oncologic rationale, surgical anatomy, and feasibility study. Am J Obstet Gynecol 1998;178:971–6.
Maas CP, DeRuiter MC, Kenter GG, Trimbos JB. The inferior hypogastric plexus in gynecologic surgery. J Gynecol Technol 1999;5:55–62.
Last RJ. Anatomy: Regional and applied. 7th ed. New York: Churchill Livingstone, 1984:344–5.
Sadler TW. Langman’s medical embryology. 5th ed. Baltimore: Williams & Wilkins, 1985:247–80.
Burnstock G, Milner P, O’Brien. Autonomic nervous system. In: Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ, eds. Gray’s anatomy. 38th ed. New York: Churchill Livingstone, 1995:1292–312.
Diokno AC, Davis R, Lapides J. The effect of pelvic nerve stimulation of detrusor contraction. Investig Urol 1973;11:178–81.
Brindley GS. The actions of parasympathetic and sympathetic nerves in human micturition, erection and seminal emission and their restoration in paraplegic patients by implanted electrical stimulators. Proc R Soc Lond B Biol Sci 1988;B235:111–20.
Fox CA, Fox B. A comparative study of coital physiology, with special reference to the sexual climax. J Reprod Fertil 1971;24:319–36.
Levin RJ. The physiology of female sexual function in women. Clin Obstet Gynecol 1980;7:213–52.
Lynch EM, Wharton J, Bryant MG, Bloom SR, Polak JH, Elder MG. The differential distribution of vasoactive intestinal peptide in the normal human female genital tract. Histochem Cell Biol 1980;67:169–70.
Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience 1976;1:239–48.
Burnstock G. Co-transmission. The fifth Heymans lecture. Arch Int Pharmacodyn Ther 1990;304:7–33.
Kupfermann I. Functional studies of co-transmission. Physiol Rev 1991;71:683–732.
Alm P, Lundberg LM. Co-existence and origin of peptidergic and adrenergic nerves in the guinea pig uterus. Retrograde tracing and immunocytochemistry, effects of chemical sympathectomy, capsaicin treatment and pregnancy. Cell Tissue Res 1988;254:517–30.
Burnstock G. Changing face of autonomic and sensory nerves in the circulation. In: Edvinsson L, Uddman R, eds. Vascular innervation and receptor mechanisms: New perspectives. Oxford: Academic Press, 1993:1–22.
Lundberg JM, Franco-Cereceda A, Hua X, Hökfelt T, Fischer JA. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol 1985;108:315–9.
Maggi CA. The pharmacology of the efferent function of cap-saicin-sensitive sensory nerves. J Auton Pharmacol 1991;11:173–208.
X-Y Hua, Theodorsson-Norheim E, Lundberg JM, Kinn AC, Hokfelt T, Cuello AC. Co-localization of tachykinins and calcitonin gene-related peptide in capsaicin-sensitive afferents in relation to motility effects on the human ureter in vitro. Neuroscience 1987;27:693–703.
Senba E, Tohyama M. Calcitonin gene-related peptide containing efferent pathways to the pelvic ganglia of the rat. J Auton Nerv Syst 1988;50:386–90.
Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory nerves. Gen Pharmacol 1988;19:1–43.
Marron K, Yacoub MH, Polak JM, et al. Innervation of human atrioventricular and arterial valves. Circulation 1996;94:368–75.
Piver SM, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol 1974;44:623–44.
Butler-Manuel SA, Buttery LDK, A’Hern RP, Polak JM, Barton DPJ. Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: The nerve content of the uterine supporting ligaments. Cancer 2000;89:834–41.
Zamboni L, De Martino C. Buffered picric acid formaldehyde: A new rapid fixative for electron microscopy. J Cell Biol 1967;35:148A.
Cohen T. Quantification of nerves and neurotransmitters using image analysis. In: Wooton R, Springall DR, Polak JM, eds. Image analysis in histology: Conventional and confocal microscopy. Cambridge, United Kingdom: Cambridge University Press, 1995:355–80.
Mokrzycki ML, Mittal K, Smilen SW, Blechman AN, Porges RF, Demopolous RI. Estrogen and progesterone receptors in the uterosacral ligament. Obstet Gynecol 1997;90:402–4.
Campbell RM. The anatomy and structure of the sacrouterine ligaments. Am J Obstet Gynecol 1950;59:1–12.
Lundberg JM, Alm P, Thorbert G. Local mechanical effects and humoral factors evoke degeneration of guinea pig uterine denervation. Acta Obstet Gynecol Scand 1989;68:487–96.
Asmussen M, Ulmsten UG. Effects of radical hysterectomy with lymph nodes dissection. Zentralbl Gynakol 1982;104:868–73.
Sasaki H, Yoshida, Noda K, Yachiku S, Minami K, Kaneko S. Urethral pressure profiles following radical hysterectomy. Obstet Gynecol 1982;59:101–4.
Sakamoto S, Takizawa K. An improved radical hysterectomy with fewer urological complications and with no loss of therapeutic results for invasive cervical cancer. Balliere’s Clin Obstet Gynaecol 1988;2:953–62.
Sakamoto S. Radical hysterectomy with pelvic lymphadenecto-my—the Tokyo method. In: Coppleson M, ed. Gynecologic oncology. 2nd ed. New York: Churchill Livingstone, 1994:1257–68.
Trimbos JB, Maas CP, Deruiter MC, Peters AAW, Kenter GG. A nerve-sparing radical hysterectomy: Guidelines and feasibility in western patients. Int J Gynecol Oncol 2001;11:180–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was presented, in part, at the 46th Annual Scientific Meeting of the Society for Gynecologic Investigation, Atlanta, Georgia, March 10–13, 1999.
The authors acknowledge the help and advice of Steven A. Corbett, PhD, FRCS, and Kevin Marron, PhD.
Rights and permissions
About this article
Cite this article
Butler-Manuel, S.A., Buttery, L.D.K., A’Hern, R.P. et al. Pelvic Nerve Plexus Trauma at Radical and Simple Hysterectomy: A Quantitative Study of Nerve Types in the Uterine Supporting Ligaments. Reprod. Sci. 9, 47–56 (2002). https://doi.org/10.1177/107155760200900110
Published:
Issue Date:
DOI: https://doi.org/10.1177/107155760200900110