Abstract
Objective
To study the regulation of the blood group A-related high-molecular weight mucin glycoprotein epitope (mouse ascites golgi, MAG)—a menstrual cycle-dependent marker of endometrial receptivity—in a non-human endometrium model.
Methods
Immature Sprague-Dawley rats were injected with 1 μg of estradiol, 100 μg of testosterone, 100 μg of dexamethasone, 2.5 mg of progesterone (P), 0.325 mg of RU486, P and RU486, 100 μg of tamoxifen, or vehicle for 3 days, sacrificed, and the uteri were stained for MAG. Immunohistochemistry and blood analysis were the measurements used to compare the specimens from the exogenous hormonal and endogenous hormonal groups. Electron microscopy was used to locate the MAG epitope in one pseudopregnant adult Sprague-Dawley rat.
Results
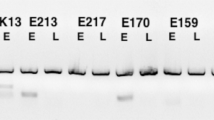
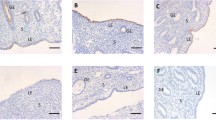
The MAG epitope was present in endometral glands of Sprague-Dawley rats, with maximal expression during proestrus and diestrus. Electron microscopy confirmed the Golgi location of this MAG epitope. In the untreated group, less than 0.5% of endometrial glands stained for MAG. The MAG was seen only in the glands of the P-treated rats and RU486 blunted this stimulatory effect by more than 95%. As little as 0.1 mg of P promoted MAG expression, with maximal response at 2.5 mg. Staining was seen 24 hours after P treatment, peaked at 72 hours, then declined. Induction of endogenous P by superovulation with pregnant mare serum gonadotropin (PMSG) and hCG (pseudopregnancy) also resulted in strong MAG glandular staining.
Conclusion
Our results suggest that the MAG epitope is cyclically expressed and induced by P in rat endometrial glands.
Similar content being viewed by others
References
Navot D, Scott RT, Droesch K, Veeck LL, Liu H-C L, Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril 1991;55:114–8.
Miller DJ, Macek MB, Shur BD. Complementarity between sperm surface B-1,4-galactosyl-transferase and egg-coat ZP3 mediates sperm-egg binding. Nature 1992;357:589–93.
Hindsgaul O, Kaur KJ, Srivastava G, et al. Evaluation of deoxygenated oligosaccharide acceptor analogs as specific inhibitors of glycosyltransferases. J Biol Chem 1991;266:17858–62.
Lindenberg S, Hyttel P, Sjogren A, Greve T. A comparative study of attachment of human, bovine, and mouse blastocysts to uterine epithelial monolayer. Hum Reprod 1989;4:446–56.
Kimber SJ, Lindenberg S. Hormonal control of a carbohydrate epitope involved in implantation in mice. J Reprod Fertil 1990; 89:13–21.
Lindenberg S. Experimental studies on the initial trophoblast endometrial interaction. Danish Med Bull 1991;38:371–80.
Kliman HJ, Feinberg RF, Schwartz LB, Feinman MA, Lavi E, Meaddough EL. A human endometrial mucin identified by MAG (mouse ascites Golgi) antibodies: Menstrual cycle-dependent localization in human endometrium. Am J Pathol 1995;146: 166–81.
Lee YH, Howe RS, Sha S-J, Teuscher C, Sheehan DM, Lyttle CR. Estrogen regulation of an eosinophilic chemotactic factor in the immature rat uterus. Endocrinol 1989;125:3022–8.
Brown-Grant K, Munck A, Naftolin F, Sherwood MR. The effects of the administration of testosterone propionate alone or with phenobarbitone and of testosterone metabolites to neonatal female rats. Horm Behav 1971;2:173–82.
Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrus cycle and early pseudopregnancy in the rat: Prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancv. Endocrinology 1975;96:219–26.
Orczyk GP, Hichens M, Arth G, Behrman HR. Progesterone and 20 alpha dihydroprogesterone. In: Jaffe BM, Behrman HR, eds. Methods of hormone radioimmunoassay. 2nd ed. New York: Academic Press, 1979:701–13.
Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide-Y innervation of B-endorphin-containing cells in the rat mediobasal hypothalamus: A light and electron microscopic double immunostaining analysis. Endocrinol 1992;131:2461–7.
Parkes AS. Marshall’s physiology of reproduction. Great Britain: Longman’s Green & Co. Ltd., 1966.
Clarke CL. Cell-specific regulation of progesterone receptor in the female reproductive system. Mol Cell Endocrinol 1990;70: C29–C33.
Enders AC, Schlafke S. A morphologic analysis of the early implantation stages in the rat. Am J Anat 1967;120:185–226.
Aplin JD. Glycans as biochemical markers of human endometrial secretory differentiation. J Reprod Fertil 1991;92:525–41.
Carson D, Ferrar JD, Laidlaw J, Wright DA. Selective activation of the N-glycosylation apparatus in uteri by estrogen. J Biol Chem 1990;265:2947–55.
Munakata H, Isemura M, Yosizawa Z. Enzymatic sulfation of exogenous high molecular weight glycopeptides by microsomal fraction of the rabbit uterine endometrium. J Biol Chem 1985; 260:6851–6.
Enders AC, Schlafke S. Surface coat of the mouse blastocyst and the uterus during the preimplantation period. Anat Rec 1974; 181:31–46.
Hewitt K, Beer AE, Grinnell F. Disappearance of anionic sites from the surface of the rat endometrial epithelium at the time of blastocyst implantation. Biol Reprod 1979;21:691–707.
Chavez DJ, Anderson TL. The glycocalyx of mouse uterine luminal epithelium during estrus, the peri-implantation period, and delayed implantation. I. Acquisition of RCA-1 binding sites during pregnancy. Biol Reprod 1985;32:1135–42.
Sato M, Muramatsu T, Berger EG. Immunological detection of cell surface galactosyltransferase in preimplantation mouse embryos. Dev Biol 1984;102:514–8.
Ghosh D, Sengupta J. Anti-nidatory effect of a single, early post-ovulatory administration of mifepristone (RU-486) in the rhesus monkey. Hum Reprod 1993;8:552–8.
Ben-Nun I, Ghetler Y, Jaffe R, Siegal A, Kaneti H, Fejgin M. Effect of preovulatory progesterone administration on the endometrial maturation and implantation rate after in vitro fertilization and embryo transfer. Fertil Steril 1990;53:276–81.
Howles CM, MacNamee MC, Edwards RG. Progesterone supplementation in the late follicular phase of an in-vitro fertilization cycle: A “natural” way to time oocyte recovery? Hum Reprod 1988;3:409–12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded in part by a ACOG/Mead Johnson Clinical Research Fellowship award (LBS).
We wish to acknowledge Dr. Harold Behrman of the Division of Reproductive Endocrinology, Department of Obstetrics and Gynecology, Yale University, for his guidance in the performance of the progesterone assays and helpful discussions, Dr. James Jamieson, Department of Cell Biology, Yale University, for his assistance in the interpretation of the electron photomicrographs, Dr. Richard Hochberg, Department of Obstetrics and Gynecology, Yale University, for supplying the RU486 used in these experiments, and Marya Shanabrough for her technical assistance in the performance of the specimen preparation for electron microscopy.
Rights and permissions
About this article
Cite this article
Schwartz, L.B., Naftolin, F., Lyttle, C.R. et al. Mouse Ascites Golgi (MAG) Mucin Expression and Regulation by Progesterone in the Rat Uterus. Reprod. Sci. 8, 216–223 (2001). https://doi.org/10.1177/107155760100800406
Published:
Issue Date:
DOI: https://doi.org/10.1177/107155760100800406