Abstract
Objective
There is increasing evidence from animal experiments that mild hypothermia induced during or after cerebral ischemia might protect the immature brain from neuronal cell damage. However, the exact interrelation between the postischemic time delay and the degree of mild hypothermia by which to achieve neuroprotective effects on ischemic insults of different severity has not yet been elucidated systematically. To determine optimal neuroprotection, we studied the intention between these variables in a recently modified hippocampal slice model.
Methods
We investigated the recovery of energy metabolism and protein synthesis (PSR) in hippocampal slices from mature fetal guinea pigs after 20, 30, or 40 minutes of oxygen and glucose deprivation (OGD). Hypothermia of varying degree was induced immediatey or 2 or 4 hours after OGD and lasted for 10 hours. Prolonged inhibition of PSR after ischemia has been shown to be a sensitive marker of neuronal cell damage.
Results
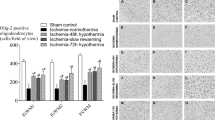
Hypothermia initiated immediately after OGD significantly improved the recovery of energy metabolism and PSR. If there was a 2-hour delay in the onset of hypothermia, neuroprotection depended on the degree of hypothermia. Reduction of the incubation temperature to 31C diminished the disturbances of energy metabolism and PSR, whereas lowering the bath temperature to only 34C was not effective. Hypothermia induced 4 hours after OGD did not have any influence on the recovery of energy metabolism and PSR.
Conclusion
We conclude that the effects of mild hypothermia on metabolic disturbances in hippocampal slices of mature fetal guinea pigs depended on the intervention delay and the degree of cooling. The shorter the postischemic intervention delay and the greater the degree of hypothermia, the better the neuroprotective effect seems to be.
Similar content being viewed by others
References
Volpe JJ. Neurology of the newborn. Philadelphia: WB Saunders Company, 1995.
Berger R, Gamier Y. Pathophysiology of perinatal brain damage. Brain Res Rev 1999;30:107–34.
Berger R, Jensen A, Hossmann KA, Paschen W. Effect of mild hypothermia during and after transient in vitro ischemia on metabolic disturbances in hippocampal slices at different stages of development. Dev Brain Res 1998;105:67–77.
Bona E, Hagberg H, Loberg EM, Bagenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxic-ischemia: Short- and long-term outcome. Pediatr Res 1998;43:738–45.
Laptook AR, Corbett RJ, Sterett R, Burns DK, Tollefsbol G, Garcia D. Modest hypothermia provides partial neuroprotection for ischemic neonatal brain. Pediatr Res 1994;35:436–42.
Sirimanne ES, Blumberg RM, Bossano D, et al. The effect of prolonged modification of cerebral temperature on outcome after hypoxic-ischemic brain injury in the infant rat. Pediatr Res 1996;39:591–7.
Thoresen M, Bagenholm R, Loberg EM, Apricena F, Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Educ 1996;74:F3–9.
Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res 1995;37:667–70.
Trescher WH, Ishiwa S, Johnston MV. Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev 1997;19:326–38.
Yager J, Towfighi J, Vannucci RC. Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res 1993;34:525–9.
Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 1997;99:248–56.
Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 1998;102:1098–106.
Carroll M, Beek O. Protection against hippocampal CA1 cell loss by post-ischemic hypothermia is dependent on delay of initiation and duration. Metab Brain Dis 1992;7:45–50.
Coimbra C, Wieloch T. Moderate hypothermia mitigates neuronal damage in the rat brain when initiated several hours following transient ischemia. Acta Neuropathol 1994;87:325–31.
Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res 1994; 654:265–72.
Colbourne F, Corbett D. Delayed post-ischemic hypothermia: A sixth month survival study using behavioral and histologic assessments of neuroprotection. J Neurosci 1995;15:7250–60.
Colbourne F, Li H, Buchan AM. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. J Cereb Blood Flow Metab 1999;19:742–9.
Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. Mol Neurobiol 1997;14:171–201.
Berger R, Djuricic B, Jensen A, Hossmann KA, Paschen W. Ontogenetic differences in energy metabolism and inhibition of protein synthesis in hippocampal slices during ischemia and 24 h of recovery. Dev Brain Res 1996;91:281–91.
Hossmann KA, Widmann R, Wiessner C, Dux E, Djuricic B, Rõhn G. Protein synthesis after global ischemia and selective vulnerability. In: Krieglstein J, Oberpichler-Schwenk H, eds. Pharmacology of cerebral ischemia. Stuttgart: Wissenschaftliche Verlagsgesellschaft mbH, 1992:289–99.
Penny JE, Kukums JR, Tyrer JH, Eadie MJ. Selective vulnerability of the hippocampus to hypoxia: Cytophotometric studies of enzyme activity in single neurons. Proc Aust Assoc Neurol 1974;11:177–81.
Djuricic B, Berger R, Paschen W. Protein synthesis and energy metabolism in hippocampal slices during extended (24 hours) recovery following different periods of ischemia. Metab Brain Dis 1994;9:379–91.
Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochem 1968;7:4030–4.
Lowry OH, Rosenbrough NJ, Farr AL, Randall R. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193:265–75.
Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res 1999;46:274–80.
Bodsch W, Takahashi K, Barbier A, Grosse Ophoff B, Hossmann KA. Cerebral protein synthesis and ischemia. Prog Brain Res 1985;63:197–210.
Thilmann R, Xie Y, Kleihues P, Kiessling M. Persistent inhibition of protein synthesis precedes delayed neuronal death in postischemic gerbil hippocampus. Acta Neuropathol 1986;71: 88–93.
Widmann R, Miyazawa T, Hossmann KA. Protective effect of hypothermia on hippocampal injury after 30 minutes of forebrain ischemia in rats is mediated by postischemic recovery of protein synthesis. J Neurochem 1993;61:200–9.
Cooper H, Zalewska T, Kawakami S, Hossmann KA. The effect of ischemia and recirculation on protein synthesis in the brain. J Neurochem 1977;28:929–34.
Hu BR, Wieloch T. Stress-induced inhibition of protein synthesis initiation: Modulation of initiation factor 2 and guanine nucleotide exchange factor activities following transient cerebral ischemia in the rat. J Neurosci 1993;13:1830–8.
Degracia DJ, Neumar RW, White BC, Krause GS. Global ischemia and reperfusion — modifications in eukaryotic initiation factors associated with inhibition of translation initiation. J Neurochem 1996;67:2005–12.
Samuel CE. The eIF-2α protein kinases, regulators of translation in eukaryotes from yeast to humans. J Biol Chem 1993;268: 7603–6.
Prostko CR, Dholakia JN, Brostrom MA, Brostrom CO. Activation of the double-stranded RNA-regulated protein kinase by depletion of endoplasmic reticulum calcium stores. J Biol Chem 1995;270:6211–5.
Srivastava SP, Davies MV, Kaufman RJ. Calcium depletion from the endoplasmic reticulum activates the double-stranded RNA-dependent protein kinase (PKR) to inhibit protein synthesis. J Biol Chem 1995;270:16619–24.
Paschen W. Disturbance of calcium homeostasis within the endoplasmic reticulum may contribute to the development of ischemic cell damage. Med Hypotheses 1996;47:283–8.
Benedikt FC, Lee RC. Hibernation and marmot physiology. Publication no. 494. Washington, DC: Carnegie Institution of Washington, 1938.
Bigelow WG, Lindsay WK, Harrison RC. Oxygen transport and utilization in dogs at low body temperatures. Am J Physiol 1950;160:125–37.
Kramer RS, Sanders AP, Lesage AM. The effect of profound hypothermia on preservation of cerebral ATP content during circulatory arrest. J Thorac Cardiovasc Surg 1968;56:699–709.
Michenfelder JD, Theye RA. Hypothermia: Effect on canine brain and whole body metabolism. Anaesthesiology 1968;29: 1107–12.
Michenfelder JD, Theye RA. The effects of anesthesia and hypothermia on canine cerebral ATP and lactate during anoxia produced by decapitation. Anaesthesiology 1970;33:430–9.
Sutton LN, Clark BJ, Norwood CR, Woodford EJ, Welsh FA. Global cerebral ischemia in piglets under conditions of mild and deep hypothermia. Stroke 1991;22:1567–73.
Swain JA, McDonald TJ Jr, Balaban RS, Robbins RC. Metabolism of the heart and brain during hypothermic cardiopulmonary bypass. Ann Thorac Surg 1991;51:105–9.
Nedelcu J, Klein MA, Aguzzi A, Martin E. Resuscitative hypothermia protects the neonatal rat brain from hypoxic-ischemic injury. Brain Pathol 2000;10:61–71.
Packianathan S, Cain CD, Liwnicz BH, Longo LD. Ornithine decarboxylase activity in vitro in response to acute hypoxia: A novel use of newborn rat brain slices. Brain Res 1995;688:61–71.
Saito K, Packianathan S, Longo LD. Free radical-induced elevation of ornithine decarboxylase activity in developing rat brain slices. Brain Res 1997;763:232–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Deutsche Forschungsgemeinschaft Be 1688/4-1.
The excellent technical assistance of Stefan Reininghaus and Bianca Lammerding is gratefully acknowledged.
Rights and permissions
About this article
Cite this article
Garnier, Y., Pfeiffer, D., Jensen, A. et al. Effects of Mild Hypothermia on Metabolic Disturbances in Fetal Hippocampal Slices After Oxygen/Glucose Deprivation Depend on Depth and Time Delay of Cooling. Reprod. Sci. 8, 198–205 (2001). https://doi.org/10.1177/107155760100800403
Published:
Issue Date:
DOI: https://doi.org/10.1177/107155760100800403