Abstract
Objective
To test the hypothesis that amnion cytokine production might be regulated by prostanoids.
Methods
Amnion-derived WISH cells were treated with a range of prostanoids and their effects on production of interleukin (IL)-6 and IL-8 were determined by enzyme-linked immunosorbent assay and Northern analysis. The effect of thromboxane inhibitors on cytokine production by term primary amnion explants also were examined.
Results
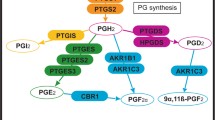
Prostaglandin (PG)A2, PGD2, PGF2a PGE2, PGJ2, and the PGI2 analogue carbaprostacyclin (1–1000 nmol/L) exhibited no significant effects on cytokine production. However, the thromboxane A2 (TXA2) agonists U46619 and carbocyclic (c)TXA2 both stimulated WISH cytokine production with similar potencies under basal or cytokine-stimulated conditions. Significant stimulation of IL-6 production was observed at concentrations ≥8 nmol/L (P < .05 by analysis of variance), whereas IL-8 production was stimulated significantly but to a lesser extent. The effects of U46619 and cTXA2 were rapid; maximal stimulation of cytokine production occurred within 4 to 8 hours of treatment. U46619 augmented IL-1βstimulated IL-6 and IL-8 mRNA expression within 2 hours of treatment. In amnion explants inhibitors of TX synthesis and action abrogated the stimulatory effect of IL-1βon cytokine production.
Conclusion
These results are consistent with the presence of a feed-forward loop in amnion involving TXA2 and cytokines, which could play a significant role in the progression of the inflammatory response involved in the mechanism of infection-driven preterm labor.
Similar content being viewed by others
References
Mitchell MD, Strickland DM, Brennecke SP, Saeed SA. New aspects of arachidonic acid metabolism and human parturition. In: MacDonald PC, Porter J, eds. Initiation of parturition: Prevention of prematurity. Report of the 4th Ross Conference on Obstetric Research. Columbus, Ohio: Ross Laboratories, 1982:145–53.
Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of preterm birth and subclinical infection. Am J Obstet Gynecol 1992;16:1515–28.
Dudley DJ, Trautman MS. Infection, inflammation and contractions: The role of cytokines in the pathophysiology of preterm labour. Semin Reprod Endocrinol 1994;12:263–72.
Mitchell MD, Branch DW, Lundin-Schiller S, Romero RJ, Daynes RA, Dudley DJ. Immunologic aspects of preterm labor. Semin Perinatol 1991;15:210–24.
Gravett MG, Witkin SS, Novy MD. A non-human primate model for chorioamnionitis and preterm labor. Semin Reprod Endocrinol 1994;12:246–62.
Mitchell MD. Eicosanoid biosynthesis and its regulation during human pregnancy and parturition. In: Thornburn GD, Harding R, eds. Textbook of fetal physiology. Oxford: Oxford University Press, 1994:430–44.
Halgunset J, Johnsen H, Kjollesdal AM, Qvigstad E, Espevik T, Austgulen R. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. Eur J Obstet Gynecol Reprod Biol 1994;56:153–60.
Romero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, Mitchell MD. Increased concentrations of arachidonic acid lipoxygenase metabolites in amniotic fluid during parturition. Obstet Gynecol 1987;70:849–51.
Romero R, Manogue KR, Mitchell MD, et al. Cachectin: Tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–41.
Opsjon SL, Wathen NC, Tingulstad S, et al. Tumour necrosis factor, intcrleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol 1993;169:397–404.
Wilhelmsson L, Wikland W, Wiqvist N. PGH2, TXA2 and PGI2have potent and differentiated actions on human uterine contractility. Prostaglandins 1981;21:277–86.
Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-a formation in human decidua. Potential role of cytokines in infection-induced preterm labour. J Clin Invest 1989;83:430–6.
Bry K, Hallman M, Lappalainen U. Cytokines released by granulocytes and mononuclear cells stimulate amnion cell prostaglandin E2 production. Prostaglandins 1994;48:389–99.
Mitchell MD, Bibby J, Hicks BR, Turnbull AC. Specific production of prostaglandin E by human amnion in vitro. Prostaglandins 1978;15:377–82.
Challis JRG, Lye Sj, Gibb W. Prostaglandins and parturition. Ann N Y Acad Sci 1997;828:254–67.
Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1990;81:941–8.
Stallmach T, Hebisch G, Joller-Jemelka HI, Orban P, Schwaller J, Engelmann M. Cytokine production and visualized effect in the feto-maternal unit. Lab Invest 1995;73:384–92.
Dudley DJ, Hunter C, Mitchell MD, Varner MW. Clinical value of amniotic fluid interleukin-6 determinations in the management of preterm labor. Br J Obstet Gynaecol 994;101:592–7.
Coultrip LL, Lien JM, Gomez R, Kapernick P, Khoury A, Grossman JH. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1994;171:901–11.
Kent As, Sullivan MH, Sun MY, Zosmer A, Elder MG. Effects of interleukin-6 and tumor necrosis factor-a on prostaglandin production by cultured human fetal membranes. Prostaglandins 1993;46:351–9.
Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells. Eur J Pharmacol 1991;192:189–91.
Kelly RW. Inflammatory mediators and parturition. Rev Reprod 1996;1:89–96.
Kunkel SL, Chensue SW, Phan SH. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol 1986;136:186–92.
Knudsen PJ, Dinarello CA, Strom TB. Prostaglandins posttranslationally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol 1986;137:3189–94.
Kunkel SL, Remick DG, Spengler M, Chensue SW. Modulation of macrophage-dcrived interleukin-1 and tumor necrosis factor by prostaglandin E2. Adv Prostaglandin Thromboxane Leukot Res 1986;17:155–8.
Delia Bella S, Molteni M, Compasso S, Zulian C, Vanoli M, Scorza R. Differential effects of cyclo-oxygenase pathway metabolites on cytokine production by T-lymphocytes. Prostaglandins Leukot Essent Fatty Acids 1997;56:177–84.
Meja KK, Barnes PJ, Giembycz MA. Characterization of the protanoid receptor(s) on human blood monocytes at which prostaglandin E2 inhibits lipopolysaccharide-induced tumour-necrosis factor-a generation. Br J Pharmacol 1997;122:149–57.
Takigawa M, Takashiba S, Takahashi K, Arai H, Kurihawa H, Murayama Y. Prostaglandin E2 inhibits interleukin-6 release but not its transcription in human gingivial fibroblasts stimulated with interleukin-1β or tumor necrosis factor-α. J Periodontol 1994;65:1122–7.
Goss JA, Mangino MJ, Gallery MP, Flye MW. Prostaglandin E2down-regulates Kupffer cell production of IL-1 and IL-6 during hepatic regeneration. Am J Physiol 1993;264:G601–8.
Standiford TJ, Kunkel SL, Rolfe MW, Evanoff HL, Allen RM, Streiter RM. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol 1992;6:75–81.
Gong J-H, Renz H, Sprenger H, Nain M, Gemsa D. Enhancement of tumor-necrosis factor-a gene expression by low doses of prostaglandin E2 and cyclic GMP. Immunobiology 1990;182:44–55.
Holliday CS, Wright RM, Spangelo BL. Arachidonic acid stimulates interleukin-6 release from rat peritoneal macrophages in vitro: Evidence for a prostacyclin-dependent mechanism. Prostaglandins Leukot Essent Fatty Acids 1993;49:915–22.
Tsuboi I, Tanaka H, Nakao M, Shichijo S, Itoh K. Non-steroidal anti-inflammatory drugs differentially regulate cytokine production in human lymphocytes: Up-regulation of TNF, IFN-γ and IL-2, in contrast to down-regulation of IL-6 production. Cytokine 1995;4:372–9.
Fiebich BL, Hull M, Lieb K, Gyufko K, Berger M, Bauer J. Prostaglandin E2 induces interleukin-6 synthesis in human astrocytoma cells. J Ncurochem 1997;68:704–9.
Renz H, Gong J-H, Schmidt A, Nain M, Gemsa D. Release of tumor necrosis factor-α from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2and cyclic nucleotides. J Immunol 1988;141:2388–93.
Bailly S, Ferrua B, Fay M, Gougerot-Pocidalo MA. Differential regulation of IL-6, IL-1α, IL-1β and TNF-α production in LPS-stimulated human monocytes: Role of cyclic AMP. Cytokine 1990;2:205–10.
Agro A, Langdon C, Smith F, Richards CD. Prostaglandin E2enhances interleukin 8 (IL-8) and IL-6 but inhibits GMCSF production by IL-1 stimulated human synovial fibroblasts in vitro. J Rheumatol 1996;23:862–8.
Wertheim WA, Kunkel SL, Standiford TJ, et al. Regulation of neutrophil-derived IL-8: The role of prostaglandin E2, dexamethasone, and IL-4. J Immunol 1993;151:2166–75.
Caughey GE, Pouliot M, Cleland LG, James MJ. Regulation of tumor necrosis factor-a and interleukin-1β synthesis by thromboxane A2 in non-adherent monocytes. J Immunol 1997;158:351–8.
Vedin I, Wasserman J, Hammarstrom S. Stimulation of tumor necrosis factor-a release from lipopolysaccharide-activated human blood monocytes by prostaglandin J2 and metabolites of prostaglandin J2. Prostaglandins Leukot Essent Fatty Acids 1997;55:185–6.
Spaziani EP, Tsibris JC, Hunt LT, Benoit RR, O’Brien WF. The effects of interleukin-1β and interleukin-4 on the expression of prostaglandin receptors EP1 and EP3 in amnion WISH cells. Am J Reprod Immunol. 1997;38:279–85.
Hayflick L. The establishment of a line (WISH) of human amnion cells in continuous cultivation. Exp Cell Res 1961;23:14–20.
Keelan JA, Sato T, Mitchell MD. Regulation of interleukin (IL)-6 and IL-8 production in an amnion-derived cell line by cytokines, growth factors, glucocorticoids, and phorbol esters. Am J Reprod Immunol 1997;38:272–8.
Redinbaugh MG, Turley RB. Adaption of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem 1986;153:267–71.
Simpson KL, Keelan JA, Mitchell MD. Labor-associated changes in interleukin-10 production and its regulation by immuno-modulators in human choriodecidua. J Clin Endocrinol Metab 1998;83:4332–7.
Keelan JA, Sato T, Mitchell MD. Interleukin (IL)-6 and IL-8 production by human amnion cells in vitro: Regulation by cytokines, growth factors, glucocorticoids, phorbol esters and bacterial lipopolysaccharide. Biol Reprod 1997;57:1438–44.
Gilmour JS, Hansen WR, Miller HC, Keelan JA, Sato TA, Mitchell MD. Effects of interleukin-4 on the expression and activity of prostaglandin endoperoxide H synthase-2 in amnion-derived WISH cells. J Mol Endocrinol 1998;21:317–25.
Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986;324:73–5.
Matsushima K, Morishita K, Yoshimura T, et al. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumour necrosis factor. J Exp Med 1988;167:1883–93.
Tso JY, Sun KH, Kao TH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldchyde-3-phosphate dehydrogenase cDNAs: Genomic complexity and molecular evolution of the gene. Nucleic Acids Res 1985;13:2485–502.
Berretini M, De Cunto M, Grassali S, Nenci GG. In vitro and ex vivo effects of picotamide, a combined thromboxane A2-synthesis inhibitor and -receptor antagonist on human platelets. Eur J Clin Pharmacol 1990;39:495–500.
Gormann RR, Johnson RA, Spilman CH, Aiken JW. Inhibition of platelet thromboxane A2 synthase activity by sodium 5-(3-pyridinylmethyl)benzofuran-2-carboxylate. Prostaglandins 1983;26:325–42,
Romero R, Baumann P, Gomez R, et al. The relationship between spontaneous rupture of membranes, labor, and microbial invasion of the amniotic cavity and amniotic fluid concentrations of prostaglandins and thromboxane B2 in term pregnancy. Am J Obstet Gynecol 1993;168:1654–68.
Romero R, Baumann P, Gonzalez R, et al. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am J Obstet Gynecol 1994;171:1613–20.
Mitchell MD, Keirse MJNC, Anderson ABM, Tumbull AC. Thromboxane B2 in amniotic fluid before and during labour. Br J Obstet Gynaecol 1978;85:442–5.
Makarainen L, Ylikorkala O. Amniotic fluid 6-keto-prostaglandin F1α and thromboxane B2 during labor. Am J Obstet Gynecol 1984;150:765–8.
Dawood MY. Non-steroidal anti-inflammatory drugs and reproduction. Am J Obstet Gynecol 1993;169:1255–65.
Dyal R, Crankshaw DJ. The effects of some synthetic prostanoids on the contractility of the lower human uterine segment in vitro. Am J Obstet Gynecol 1988;158:281–5.
Senior J, Sangha R, Baxter GS, Marshall K, Clayton JK. In vitro characterization of prostanoid FP-, DP-, IP- and TP-receptors on the non-pregnant human myometrium. Br J Pharmacol 1993;108:501–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was funded by grants from the Health Research Council of New Zealand, the New Zealand Lottery Health Grants Board, and the Auckland Medical Research Foundation.
The authors thank the nursing staff at National Women’s Hospital, Auckland, for help in assisting with the collection of tissues; Dr. N. Onai, Department of Preventative Medicine, University of Tokyo, for the interleukin (IL)-8 cDNA; and Dr. T. Fukuda, Osaka University Medical School, for the gift of the IL-6 cDNA.
Rights and permissions
About this article
Cite this article
Keelan, J.A., Sato, T.A., Gupta, D.K. et al. Prostanoid Stimulation of Cytokine Production in an Amnion-Derived Cell Line: Evidence of a Feed-Forward Mechanism With Implications for Term and Preterm Labor. Reprod. Sci. 7, 37–44 (2000). https://doi.org/10.1177/107155760000700106
Published:
Issue Date:
DOI: https://doi.org/10.1177/107155760000700106