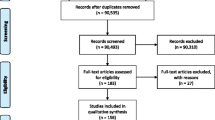
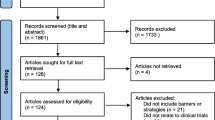
Abstract
This paper presents the background to the DIA Workshop “Clinical Trials and GCP in East Asia,” in which experts from China, Japan, Republic of Korea, Singapore, and Taiwan participated. Common features and differences among these five countries are discussed. A brief historical review of Good Clinical Practice (GCP) is provided, including some recent developments in the region. Some recent forums in Asia relating to clinical trials and GCP are introduced and ethical issues involved in clinical trials are addressed from the socio-cultural context, particularly the different ethical principles in the East Asian region and the West. Cross-national ethics in the use of foreign clinical trial data are also addressed.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsutani, K. General View of Clinical Trials and GCP in East Asia. Ther Innov Regul Sci 31, 1057–1064 (1997). https://doi.org/10.1177/009286159703100402
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286159703100402