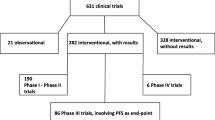
Abstract
Use of blinded independent central review (BICR) has become more common in oncology phase 3 trials as progression-free survival (PFS) has been increasingly used as an endpoint for regulatory approval. Since PFS is primarily a radiographic endpoint, BICR has been implemented to assess and reduce potential bias in the local evaluation (LE) of PFS. Recent publications note an agreement between LE and BICR of the ultimate reported PFS treatment effect, which questions the need for costly and time-consuming complete-case BICR of PFS. A meta-analysis was conducted to systematically evaluate the relationship between BICR- and LE-assessed PFS based on FDA’s regulatory experience from 2005 to present. Our results support the claim that a complete review of all radiographs by BICR may not be necessary for oncology trials, and alternative methods should be explored to evaluate bias. One potential alternative is to use BICR as an audit tool to detect evaluation bias in LE assessments.
Similar content being viewed by others
References
Amit O, Bushnell W, Dodd L, Roach N, Sargent D. Blinded independent central review of the progression-free survival endpoint. Oncologist. 2010;15:492–495.
Dodd LE, Korn EL, Freidlin B, et al. Blinded independent central review of progression-free survival in phase III clinical trials: important design element or unnecessary expense? J Clin Oncol. 2008;26:3791–3796.
Dancey JE, Dodd LE, Ford R, et al. Recommendations for the assessment of progression in randomized cancer treatment trials. Eur J Cancer. 2009;45:281–289.
Stone AM, Bushnell W, Denne J, et al. Research outcomes and recommendations for the assessment of progression in cancer clinical trials from a PhRMA working group. Eur J Cancer. 2011;47:1763–1771.
Amit O, Mannino F, Stone AM, et al. Blinded independent central review of progression in cancer clinical trials: results from a meta-analysis. Eur J Cancer. 2011;47:1772–1778.
Pogue J, Walter SD, Yusuf S. Evaluating the benefit of event adjudication of cardiovascular outcomes in large sample RCTs. Clin Trials. 2009;6:239–251.
Pignatti F, Hemmings R, Jonsson B. Is it time to abandon complete blinded independent central radiological evaluation of progression in registration trials? Eur J Cancer. 2011;47:1759–1762.
Dodd LE, Korn EL, Freidlin B, Gray R, Bhattacharya S. An audit strategy for progression-free survival. Biometrics. 2011;67:1092–1099.
Korn EL, Dodd LE, Freidlin B. Measurement error in the timing of events: effect on survival analyses in randomized clinical trials. Clin Trials. 2010;7:626–633.
Fleischer F, Gaschler-Markefski B, Bluhmki E. How is retrospective independent review influenced by investigator-introduced information censoring: a quantitative approach. Stat Med. 2011;30:3373–3386.
Oncologic Drug Advisory Committee. April 12, 2011 Meeting. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/ucm235829.htm. Accessed April 18, 2012.
Tang PA, Pond GR, Chen EX. Influence of an independent review committee on assessment of response rate and progression-free survival in phase III clinical trials. Ann Oncol. 2010;21:19–26.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article reflects the views of the authors and should not be construed to represent the US Food and Drug Administration’s views or policies.
Rights and permissions
About this article
Cite this article
Zhang, J.J., Chen, H., He, K. et al. Evaluation of Blinded Independent Central Review of Tumor Progression in Oncology Clinical Trials: A Meta-analysis. Ther Innov Regul Sci 47, 167–174 (2013). https://doi.org/10.1177/0092861512459733
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/0092861512459733