Abstract
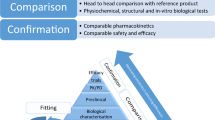
After years of public debate, biosimilars and interchangeable biological products are closer to becoming reality for patients in the United States. President Obama’s health care reform bill amends the PHS Act to strike a balance between “innovation and consumer interests.” This article provides the highlights of this legislation and includes, among other topics, the accepted and established scientific and regulatory principles supporting decision making for these products; marketing exclusivity for the innovator’s reference product and the first approved interchangeable product; activities and timelines to foster communication to avoid lengthy discussion and court action in regard to patent infringement; and administrative issues, such as naming, guidance documents, and user fees.
Similar content being viewed by others
References
Patient Protection and Affordable Health Care Act of 2010. http://democrats.senate.gov/reform/patient-protection-affordable-care-act-as-passed.pdf. Accessed September 9, 2010.
US Food and Drug Administration. The Public-Health Service Act. http://www.fda.gov/RegulatoryInformation/Legislation/ucm148717.htm. Accessed September 9, 2010.
The Food. Drug and Cosmetics Act. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/FDCActChapterVDrugsandDevices/ucm108125.htm. Accessed September 9, 2010.
Woodcock J, Griffin J, Behrman R, et al. The FDA’s assessment of follow-on protein products: a historical perspective. Nat Rev Drug Discov. 2007;6:437–442.
FDA guidance for industry: demonstration of comparability of human biological products, including therapeutic biotechnology-derived products. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122879.htm. Accessed September 9, 2010.
FDA guidance for industry: changes to an approved application for specified biotechnology and specified synthetic biological products. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM124805.pdf. Accessed September 9, 2010.
Sensabaugh SM. Biological generics: a business case. J Gen Med. 2007:1–14.
Commission Directive 2003/63/EC of 25 June 2003 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use. http://www.ema.europa.eu/pdfs/human/pmf/2003-63-EC.pdf. Accessed September 9, 2010.
European Public Assessment Report. Omnitrope. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Summary_for_the_public/human/000607/WC500043689.pdf. Accessed September 8, 2010.
European Public Assessment Report. Valtropin. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Summary_for_the_public/human/000602/WC500047156.pdf. Accessed September 8, 2010.
Perry G. Biosimilar medicines: towards global development and monoclonal antibodies. 7th EGA Annual Symposium on Biosimilar Medicines, April 23–24, 2009. http://www.egagenerics.com/doc/ega_biosimilars09_perry.pdf. Accessed September 6, 2010.
European Medicines Association Committee for Medicinal Products for Human Use. Summary of opinion (initial authorization). Nivestim. March 18. 2010. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001142/human_med_001344.jsp&murl=menus/medicines/medicines.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124. Accessed September 6, 2010.
European Medicines Association Committee for Medicinal Products for Human Use. Draft guideline on similar biological medicinal products containing monoclonal antibodies. November 26, 2010. http://www.ema.europa.eu/ema/doc_index.jsp?curl=pages/includes/document/document_detail.jsp?webContentlD-WC500099361&murl=menus/news_and_events/news_and_events.jsp&mid-0b01ac058009a3dc. Accessed January 4, 2011.
Kox S. The EU market environment for biosimilar medicines. 12th Annual IGPA Conference. October 1, 2009. http://www.egagenerics.com/doc/ega_presentation_Kox_IGPA%2009.pdf. Accessed September 6, 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sensabaugh, S.M. Requirements for Biosimilars and Interchangeable Biological Drugs in the United States—in Plain Language. Ther Innov Regul Sci 45, 155–162 (2011). https://doi.org/10.1177/009286151104500210
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286151104500210