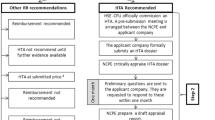
Abstract
The purpose of this article is to raise a possible solution for both reimbursement body and pharmaceutical manufacturer in situations where the price of a new drug was set too low and there is not enough incentive for the manufacturer to conduct a pharmacoeconomic (PE) or outcomes study in the local environment. For breakthrough or substantially improved products, parallel submission may reduce the time lag between marketing approval and reimbursement decision. Providing a median price of 10 reference countries could give an incentive for a manufacturer to invest in a local PE study, provide local outcomes data, and receive a price adjustment accordingly at the fourth year. This is a concept and real implementation may need more input from all stakeholders.
Similar content being viewed by others
References
Hutton J, Trueman P, Henshall C. Coverage with evidence development: an examination of conceptual and policy issues. Int J Technol Assess Health Care. 2007;23(4):425–435.
Guidance for the public, industry, and CMS staff. National coverage determinations with data collection as a condition of coverage: coverage with evidence development. Document issued July 12, 2006. Available at: http://www.cms.hhs.gov/CoverageGenlnfo/03_CED.asp/CoverageGenlnfo/03_CED.asp.
Rawlins M. Can you clarify the role and function of NICE? Nat Rev Drug Discovery. 2006;5:276. Available at: http://www.nature.com/nrd/journal/v5/n4/full/nrd2016.html/nrd/journal/v5/n4/full/nrd2016.html.
Working Group on Therapeutic Improvement. Report of the Working Group on Therapeutic Improvement to the Patented Medicine Prices Review Board. April 2008. Available at: http://www.pmprb-cepmb.gc.ca/CMFiles/Report_WG-Therapeutic_lmprovement_-_April_0838FJI-4182008–5990.pdf/CMFiles/Report_WG-Therapeutic_lmprovement_-_April_0838FJI-4182008–5990.pdf.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarn, YH., Chern, HD. A Proposal for a Parallel Submission Process for New Drug Applications and Health Technology Assessment in Taiwan: A Win-Win Solution. Ther Innov Regul Sci 43, 319–323 (2009). https://doi.org/10.1177/009286150904300311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286150904300311