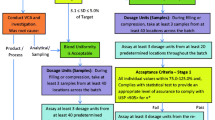
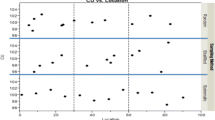
Abstract
Spectroscopic methods such as near infrared (NIR) for content uniformity (CU) allow the measurement of significantly more dosage units per batch than required by traditional pharmacopoeial testing of 10 to 30 units. Sandell et al. proposed a nonparametric counting test for sample sizes greater than 100. There are manufacturing situations such as small batch sizes or restrictions due to sampling equipment that will yield medium sample sizes between 30 and 100 units per batch. This work provides alternative CU tests for medium sample sizes.
Similar content being viewed by others
References
US Pharmacopeia Convention. Uniformity of dosage units. In: United States Pharmacopeia. Rockville, MD: United States Pharmacopeia Convention; 2005:2505–2510.
Foust L, Diener M, Gorko MA, et al. Overcoming disincentives to process understanding in the pharmaceutical CMC environment. Pharm Tech. 2007;31 (9):108–115.
Sandell D, Vukovinsky K, Diener M, Hofer J, Pazdan J, Timmermans J. Development of a content uniformity test suitable for large sample sizes. Drug Inf J. 2006;40:337–344.
Lieberman GJ, Resnikoff GJ. Sampling plans for inspection by variables. J Am Stat Assoc. 1955;50:457–516.
Harter HL, Balakrishnan N. CRC Handbook of Tables for the Use of Order Statistics in Estimation. Boca Raton. FL; CRC Press: 1996.
Bergum J. Li H. Acceptance limits for the new ICH USP 29 content uniformity test. Pharm Tech. 2007;31 (10):90–100.
Tsong Y, Shen M. Parametric two-stage sequential quality assurance test of dose content uniformity. J Biopharm Stat. 2007;17(1):143–157.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diener, M., Larner, G., Pazdan, J. et al. Development of a Content Uniformity Test Suitable for Sample Sizes between 30 and 100. Ther Innov Regul Sci 43, 287–298 (2009). https://doi.org/10.1177/009286150904300306
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286150904300306