Abstract
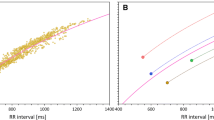
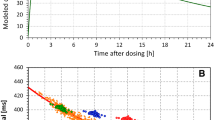
We explore adaptive (data-driven) approaches in the analysis of clinical QT data in order to find scientifically-sound solutions for correcting the QT interval for heart rate and for analysis of extreme QT measurements. We demonstrate that predefined QT correction formulas (eg, Bazett and Fridericia formulas) are unreliable when the investigational drug induces heart rate changes. Further, simple data-driven approaches (eg, QT correction formulas derived from baseline data in clinical trials) lead to a substantial inflation of the false positive rate. We discuss a QT interval analysis framework based on repeated-measures models that account for correlation among serial ECG measurements collected on the same subject and drug-induced heart rate changes. We also assess the performance of reference ranges for QT interval currently used in clinical trials.
Similar content being viewed by others
References
Algra A, Tijssen IG, Roelandt JR, Pool I, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894.
Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for Torsades de Pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597.
Malik M. Problems of heart rate correction in the assessment of drug-induced QT interval prolongation. J Cardiovascular Electrophysiology. 2001; 12.
Bazett HC. An analysis of time relations of electrocardiograms. Heart. 1920;7:353–367.
Fridericia LS. The duration of systole in the electrocardiogram of normal subjects and of patients with heart disease. Acta Medica Scandinavica. 1920;64: 469–486.
Fenichel R, Koerner J. Development of drugs that alter ventricular repolarization. 1999. http://www.fenichel.net.
Morganroth J, Brozovich FV, McDonald JT, Jacobs RA. Variability of the QT measurements in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiology. 1991;67:774–776.
Morganroth J, Brown AM, Critz S, Crumb WJ, Kunze DL, Lacerda AE, Lopez H. Variability in the QTc interval: impact on defining drug effect and low-frequency cardiac events. Am J Cardiology. 1993;64:26B–31B.
Lande G, Funck-Brentano C, Ghadanfar M, Escande D. Steady-state versus nonsteady-state QT-RR relationships in 24-hour Holter recordings. Pacing Clinical Electrophysiology. 2000;23:293–302.
Spence S, Soper K, Hoe CM, Coleman I. The heart rate-corrected QT interval of conscious beagle dogs: a formula based on analysis of covariance. Toxico-logical Sciences. 1998;45:247–258.
Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A, Shah R. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. European Heart J. 2000;21:1216–1231.
Committee for Proprietary Medicinal Products. Points to consider: The assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. London, UK: Committee for Proprietary Medicinal Products; December 17, 1997.
Health Canada. Therapeutic Products Programme. Assessment of the QT prolongation potential of non-cardiovascular drugs. Ottawa, Canada: Health Canada; March 15, 2001.
Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiology. 1992;70:797–801.
Hodges M, Salemo D, Erlien D. Bazett’s QT correction reviewed: evidence that a linear correction for heart rate is better. J Am College Cardiology. 1983;64:694.
Kawataki M, Kashima T, Toda H, Tanaka H. Relation between QT interval and heart rate applications and limitations of Bazett’s formula. J Elecrocardiology. 1984;17:371–375.
Malik M. If Dr. Bazett had had a computer.... Pacing Clinical Electrophysiology. 1996;19:1635–1639.
Vandenhende F. Heart rate specific reference ranges for QT interval in beagle dogs. Drug InfJ. 2001;64.
FDA Psychopharmacological Drugs Advisory Committee 15 February 2001. Briefing Document for Ziprasidone Mesylate for intramuscular injection. http://www.fda.gov/ohrms/dockets/ac/01/briefing/3685b2_01_pfizer.pdf.
Molnar J, Weiss J, Zhang F, Rosenthal JE. Evaluation of five QT correction formulas using a software-assisted method of continuous QT measurement from 24-hour Holter recordings. Am J. Cardiology. 1996;78:920–926.
Chuang-Stein C. Safety analysis in controlled clinical trials. Drug Inf J. 1998; 32, 1363S–1372S.
Moss AJ, Robinson JL. The long-QT syndrome: genetic considerations. Trends Cardiovascular Med. 1992;2:81–83.
MacFarlane PW, Lawie TDV. Comprehensive Electrocardiography: Theory and Practice in Health and Disease. Oxford: Pergamon Press; 1989.
FDA Psychopharmacological Drugs Advisory Committee 19 July 2000. Briefing Document for Zeldox capsules (Ziprasidone HC1). http://www.fda.gov/ohrms/dockets/ac/00/backgrd/3619bla.pdf.
FDA Anti-Infective Drugs Advisory Committee April 26, 2001. Briefing Document for Ketek (telithromycin). http://www.fda.gov/ohrms/dockets/ac/01/briefing/3746b_02_FDA.pdf.
De Bruyne MC, Hoes AW, Kors JA, Dekker JM, Hofman A, van Bemmel JH, Grobbee DE. Prolonged QT interval: a tricky diagnosis? Am J Cardiology. 1997;80:1300–1304.
Nickens DJ. Using tolerance limits to evaluate laboratory data. Drug Inf J. 1998;32:261–269.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dmitrienko, A., Smith, B.P. Analysis of The QT Interval in Clinical Trials. Ther Innov Regul Sci 36, 269–279 (2002). https://doi.org/10.1177/009286150203600205
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286150203600205