Abstract
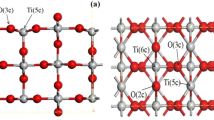
Several possible adsorption sites and adsorption geometries of CO2 on small rutile fragments were studied by Extended Hückel Molecular Orbital (EHMO) calculations. The parameters for the rutile part were optimised to reproduce the experimental rutile bulk structure and were tested in several small clusters up to [(TiO2)31(OH)32]32−•6H2O, a 175 atoms cluster. It was found that the average experimental bond legth can be reproduced with good accuracy. However the slight distortion of the TiO6 octahedra is calculated with the wrong sign (four long and two short Ti−O bonds). The agreement for the angle αO-Ti-O is less satisfactory. The study shows that CO2 can adsorb on fivefold coordinated surface titanium sites as well as surface oxygen sites. This means that CO2 can act as either Lewis base or acid. In the case of binding as a Lewis base, CO2 can adsorb linearly forming a single Ti−OCO bond, or interact with two neighboring Ti4+ sites. A chelating structure forming two Ti−O bonds was found to be weakly stable at the most. When CO2 behaves as a Lewis acid, a carbonate-like structure is formed by interaction with either terminal oxygen ions or bridging oxygen centers.
Similar content being viewed by others
References
M. Anpo and Y. Kubokawa, Res. Chem. Intermed. 8 105 (1987).
M. Anpo, Res. Chem. Intermed. 11, 67 (1989).
M. Anpo et al., Bull. Chem. Soc. Jpn. 64, 543 (1991).
N. Serpone, R. Terzian, D. Lawless, and J.-M. Herrmann, Adv. Electron Transfer Chemistry 3, 33 (1993).
A.L. Linsebigler, G. Lu, and J.T. Yates, Jr., Chem. Rev. 95, 735 (1995).
A. Fujishima and K. Honda, Nature 238, 37 (1972).
A.J. Bard and M.A. Fox, Acc. Chem. Res. 28, 141 (1995).
M. Anpo and K. Chiba, J. Mol. Catal. 74, 207 (1992).
K. Ogura, M. Kawano, J. Yano, and Y. Sakata, J. Photochem. Photobiol. A: Chem. 66, 91 (1992).
H. Yamashita, N. Kamada, H. He, K. Tanaka, and M. Anpo, Chem. Lett. 855 (1994).
H. Yamashita et al., Energy Convers. Mgmt 36, 617 (1995).
F. Saladin, L. Forss, and I. Kamber, J. Chem. Soc., Chem. Commun. 533 (1995).
F. Saladin, A. Meier, and I. Kamber, Rev. Sci. Instrum. 67, 2406 (1996).
T. Bredow and K. Jug, Chem. Phys. Lett. 223, 89 (1994).
J. Burdett, Inorg. Chem. 24, 2244 (1985).
C.-R. Wang and Y.-S. Xu, Surf. Sci. 219, L537 (1989).
B. Viswanathan and T. Lakshmi, Indian J. Chem. A 32, 937 (1993).
T. Bredow and K. Jug, Surf. Sci. 327, 398 (1995).
H. Takaba et al., Energy Convers. Magmt 36, 439 (1995).
D.C. Sayle, R.A. Catlow, M.-A. Perrin, and P. Nortier, J. Phys. Chem. Solids 56, 799 (1995).
V.E. Henrich and P.A. Cox, The Surface Science of Metal Oxides, Cambridge University Press, UK, 1994.
R.J.D. Miller et al., Surface Electron Transfer Processes, VCH Publishers, New York, 1995.
G. Calzaferri and M. Brändle, QCMP Bull. 12 (1992), update May 1993.
J. Howell et al., ICON8 quantum chemistry program performing extended-Hückel calculation, QCPE No. 344, 1978.
A.B. Anderson and R. Hoffmann, J. Phys. Chem. 60, 4271 (1974).
G. Calzaferri, L. Forss, and I. Kamber, J. Phys. Chem. 93, 5366 (1989).
F. Savary, J. Weber, and G. Calzaferri, J. Phys. Chem. 97, 3722 (1993).
N. Fitzpatrick and G. Murphy, Inorg. Chem. Acta 87, 41 (1984).
H. Basch, A. Viste, and H. Gray, Theoret. Chim. Acta (Berl.) 3, 458 (1965).
H. Basch and H. Gray, Theoret. Chim. Acta (Berl.) 4, 367 (1966).
I. Kamber, ICON-UTILS — A Collection of Utilities to Visualize the Output of EHMO Calculations, 1996.
Geomview, Version 1.5, Software Development Group, Geometry Center, Minneapolis, US, 1994, available for free at geom.umn.edu.
Goodenough and Hamnett, Landolt-Börnstein, New Series III 17g.
Y. Wang. In: Photophysical and Photochemical Processes of Semiconductor Nanoclusters, Vol. 19 of Adv. Photochem., D.C. Neckers, D.H. Volman, and G. von Brünau (Eds.), John Wiley & Sons, Inc., New York, 1995, pp. 179–234.
R. Hoffmann, Solids and Surfaces: A Chemist's View of Bonding in Extended Structures, VCH Verlagsgesellschaft mbH, Weinheim, 1988.
B. Poumellec, P. Durham, and G.Y. Guo, J. Phys.: Condens. Matter. 3, 8195 (1993).
G. Ramis, G. Busca, and V. Lorenzelli, Mater. Chem. Phys. 29, 425 (1991).
J. Raskó and F. Solymosi, J. Phys. Chem. 98, 7147 (1994).
M.N. Burnett and C.K. Johnson, ORTEP-III: Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations, 1996, Oak Ridge National Laboratory Report ORNL-6895.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kamber, I. Interaction of CO2 with small rutile crystallites-an EHMO study. Res. Chem. Intermed. 23, 735–753 (1997). https://doi.org/10.1163/156856797X00510
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856797X00510