Abstract
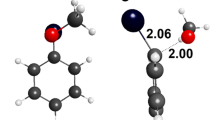
The hydrolysis of 8-bromomethyl[2.2]metacyclophanes 3 to the corresponding 8-hydroxymethyl derivatives 4 was carried out in 83% aqueous dioxane solution at 25°C. Substituent effect through space on the rate of the hydrolysis of bromomethyl groups attached on the opposite aromatic ring was first found in this investigation. Interestingly, the introduction of the substituents at the internal position 16 tends to enhance the hydrolysis reaction rate 10–100 times. It was found also that the stabilization by both the direct through-space cation-π-interaction and the interaction through the intra-annular 8,16-position are possible in the [2.2]metacyclophane 8-benzyl cations. The good correlation with log(K/KH) and σp + was observed for the hydrolysis of internally unsubstituted 5-bromomethyl[2.2]MCPs 7, in which the direct through-space cation-π-interactions are not possible.
TiCl4 and Nafion-H, a perfluorinated resinsulfonic acid, catalysed Friedel-Crafts benzylation of benzene and substituted benzenes with 8-bromomethyl- and 8-hydroxymethyl[2.2]metacyclophanes to afford 8-benzyl[2.2]metacyclophanes is described. A high substrate and positional selectivity were observed in the present benzylation reaction quite different from those obtained from the benzyl bromide and benzyl alcohol. The benzyl cation intermediate stabilized by the through-space electronic interaction among the opposite benzene ring was first demonstrated in the benzylation of [2.2]metacyclophane systems.
The mild and selective transannular reaction attributable to the highly strained character of [2.2]metacyclophane skeleton and the increased stabilization of the 5-benzyl cation intermediate arising from the electronic interactions among the opposite benzene ring through the intra-annular 8,16-positions was also observed.
Similar content being viewed by others
References
Part 42, T. Yamato, J. Matsumoto, M. Sato, K. Fujita, Y. Nagano, and M. Tashiro, submitted to J. Chem. Research (S).
C.J. Brown, J. Chem. Soc. 3278 (1953).
C.-F. Shieh, D. McNally, and R.H. Boyd, Tetrahedron 25, 3653 (1969).
A.W. Hanson, Acta Crystallor. 15, 956 (1962).
(a) P.M. Keehn and S.M. Rosenfield, Cyclophanes, Academic Press, New York, 1983; (b) D.J. Wilson, V. Boekelheide, and R.W. Griffin, Jr., J. Am. Chem. Soc. 82, 6302 (1960); (c) N.L. Allinger, M.A. Da Rooge, and R.B. Hermann, J. Am. Chem. Soc. 83, 1974 (1961); (d) H.S. Gutowsky and C. Juan, J. Chem. Phys. 37, 120 (1962); (e) T. Sato, S. Akabori, M. Kainosho, and K. Hata, Bull. Chem. Soc. Jpn 39, 856 (1966); (f) T. Sato, S. Akabori, M. Kainosho, and K. Hata, Bull. Chem. Soc. Jpn 41, 218 (1968); (g) M. Fujimoto, T. Sato, and K. Hata, Bull. Chem. Soc. Jpn 40, 600 (1967); (h) K. Burri and W. Jenny, Helv. Chim. Acta. 50, 1978 (1967); (i) N.L. Allinger, B.J. Gordon, S.-E. Hu, and R.A. Ford, J. Org. Chem. 32, 2272 (1967); (j) R. Flammang, H.P. Figeys, and R.H. Martin, Tetrahedron 24, 1171 (1968); (k) H. Blaschke, C.E. Ramey, I. Calder, and V. Boekelheide, J. Am. Chem. Soc. 92, 3675 (1970).
W.S. Lindsay, P. Stokes, L.G. Humber, and V. Boekelheide, J. Am. Chem. Soc. 83, 943 (1961).
V. Boekelheide, C. Ramey, E. Sturm, T. Miyasaka, and B.A. Hess, Jr., J. Org. Chem. 34, 1956 (1969).
Reviews: (a) R.W. Griffin, Jr., Chem. Rev. 63, 45 (1963); (b) B.H. Smith, Bridged Aromatic Compounds, Academic Press, New York, N.Y., 1964.
(a) N.L. Allinger, B.J. Gordon, H.-E. Hu, and R.A. Ford, J. Org. Chem. 32, 2272 (1967); (b) T. Sato, E. Yamada, Y. Okamura, T. Amada, and K. Hata, Bull. Chem. Soc. Jap. 38, 1049 (1965); (c) M. Fujimoto, T. Sato, and K. Hata, Bull. Chem. Soc. Jap. 40, 600 (1967); (d) T. Sato, M. Wakabayashi, Y. Okamura, T. Amada, and K. Hata, Bull. Chem. Soc. Jap. 40, 2363 (1967).
T. Sato and K. Nishiyama, J. Chem. Soc., Chem. Comm. 220 (1973).
T. Sato, K. Nishiyama, S. Shimada, and K. Hata, Bull. Chem. Soc. Jap. 44, 2858 (1971).
(a) S. Hayashi and T. Sato, Bull. Chem. Soc. Jap. 45, 2360 (1972); (b) T. Sato and K. Nishiyama, J. Org. Chem. 37, 3254 (1972).
(a) T. Yamato, S. Ide, K. Tokuhisa, and M. Tashiro, J. Org. Chem. 57, 271 (1992); (b) T. Yamato, J. Matsumoto, S. Ide, K. Tokuhisa, K. Suehiro, and M. Tashiro, J. Chem. Research (S), 420 (1992).
(a) M. Tashiro and T. Yamato, Synthesis 435 (1978); (b) M. Tashiro and T. Yamato, J. Org. Chem. 46, 1543 (1981).
(a) M. Tashiro and T. Yamato, J. Org. Chem. 46, 4556 (1981); (b) M. Tashiro and T. Yamato, Chem. Lett. 61 (1982); (c) M. Tashiro and T. Yamato, J. Chem. Soc., Perkin Trans. 1, 2165 (1984).
W.S. Lindsey, P. Stokes, L.G. Humber, and V. Boeckelheide, J. Am. Chem. Soc. 83, 943 (1961).
L.P. Hammett, J. Am. Chem. Soc. 59, 96 (1937).
G.A. Olah, Acc. Chem. Res. 4, 240 (1971).
(a) T. Yamato, N. Sakaue, C. Hideshima, and M. Tashiro, Chem. Express 5, 773 (1990); (b) T. Yamato, C. Hideshima, G.K.S. Prakash, and G.A. Olah, J. Org. Chem. 56, 2089 (1991).
(a) G.A. Olah, P.S. Iyer, and G.K.S. Prakash, Synthesis 513 (1986); (b) T. Yamato, Yukigousei Kagaku Kyoukai-shi, 53, 487 (1995) and references therein.
(a) T. Yamato, N. Hideshima, T. Furusawa, M. Tashiro, G.K.S. Prakash, and G.A. Olah, J. Chem. Res. (1991), (S), 242; (M) 2414; (b) T. Yamato, L.K. Doamekpor, H. Tsuzuki, and M. Tashiro, Chem. Lett. 89 (1995).
(a) G.A. Olah, S.J. Kuhn, and S.H. Flood, J. Am. Chem. Soc. 84, 1688 (1962); (b) G.A. Olah, S. Kobayashi, and M. Tashiro, J. Am. Chem. Soc. 94, 7448 (1972).
M. Tashiro, S. Mataka, Y. Takezaki, M. Takeshita, T. Arimura, A. Tsuge, and T. Yamato, J. Org. Chem. 54, 451 (1989).
M. Tashiro, T. Yamato, K. Kobayashi, and T. Arimura, J. Org. Chem. 52, 3196 (1987).
T. Sato, K. Torizuka, R. Komaki, and H. Atobe, J. Chem. Soc., Perkin Trans. 2 561 (1980).
Author information
Authors and Affiliations
Additional information
Parts of the present paper have been published as a preliminary communication: M. Tahiro, T. Arimura, and T. Yamato, Chem. Pharm. Bull. 31, 370 (1983).
Rights and permissions
About this article
Cite this article
Yamato, T., Fujita, K., Shinoda, N. et al. Through-space electronic interactions of [2.2]metacyclophane benzyl cations. Res. Chem. Intermed. 22, 871–897 (1996). https://doi.org/10.1163/156856796X00539
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856796X00539