Abstract
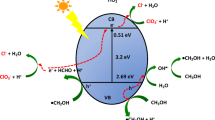
Studies on photo-catalytic reduction of CO2 using TiO2 photo-catalyst (0.1%, w/v) as a suspension in water was carried out at 350 nm light. CO2 from both commercially available source, as well as generated in situ through 2-propanol oxidation, was used for this study. The photolytic products such as hydrogen (H2), carbon monoxide (CO) andmethane (CH4) generated were monitored in TiO2 suspended aqueous solution with and without a hole scavenger, viz., 2-propanol. Similar photolytic experiments were also carried out with varying ambient such as air, O2, N2 and N2O. The yields of CO and CH4 in all these systems under the present experimental conditions were found to be increasing with light exposure time. H2 yield in N2-purged systems containing 2-propanol was found to be more as compared to the without 2-propanol system. The rate of H2 production in N2-purged aqueous solutions containing 0.1% TiO2 suspension were evaluated to be 0.226 and 5.8 μl/h, without and with 0.5 M 2-propanol, respectively. This confirmed that 2-propanol was an efficient hole scavenger and it scavenged photo-generated holes (h+), allowing its counter ion, viz., e−, to react with water molecule/H+ to yield more H2. The formation of both CO and CH4 in the photolysis of CO2-purged aqueous solutions containing suspended TiO2 in absence of 2-propanol reveal that the generation of CH4 is taking place mainly through CO intermediate. In presence of air/O2, the yield of H2 in the system without 2-propanol was observed to be negligible as compared to the system containing 2-propanol in which low yield of H2 was obtained with a formation rate of approx. 0.5 μl/h.
Similar content being viewed by others
References
G. R. Dey and K. K. Pushpa, Res. Chem. Intermed. 32, 725 (2006).
G. R. Dey, A. D. Belapurkar and K. Kishore, J. Photochem. Photobiol. A: Chem. 163, 501 (2004).
G. R. Dey and K. Kishore, in: Photo/Electrochemistry & Photobiology in the Environment, Energy and Fuel (PE&PB in EEF), S. Kaneco (Ed.), p.357. Research Signpost, Kerala (2005).
R. Memming, Top. Curr. Chem. 143, 79 (1988).
D. W. Bahnemann, in: Photochemical Conversion and Storage of Solar Energy, E. Pelizzetti and M. Schiavello (Eds), p. 251. Kluwer, Dordrecht (1991).
G. Mills and M. R. Hoffmann, Environ. Sci. Technol. 27, 1681 (1993).
M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev. 95, 69 (1995).
B. C. Faust, M. R. Hoffmann and D. W. Bahnemann, J. Phys. Chem. 93, 6371 (1989).
Y. Mao, C. Schoneich and K.-D. Asmus, J. Phys. Chem. 95, 10080 (1991).
K. Tanaka, T. Hisanaga and A. P. Rivera, in: Photocatalytic Purification and Treatment of Water and Air, H. Al-Ekabi and D. F. Ollis (Eds), p. 169. Elsevier, Amsterdam (1993).
M. Anpo, Res. Chem. Intermed. 11, 67 (1989).
A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Phtobiol. C: Photochem. Rev. 1, 1 (2000).
T. Inoue, A. Fujishima, S. Konishi and K. Honda, Nature 277, 637 (1979).
S. Kaneco, H. Kurimoto, K. Ohta, T. Mizuno and A. Saji, J. Photochem. Photobiol. A: Chem. 109, 59 (1997).
S. Kaneco, Y. Shimizu, K. Ohta and T. Mizuno, J. Photochem. Photobiol. A: Chem. 115, 223 (1998).
H. Noda, S. Ikeda, Y. Oda, K. Imai, M. Maeda and K. Ito, Bull. Chem. Soc. Jpn. 63, 2459 (1990).
R. G. Russel, N. Novac, S. Srinivasan and M. Steinberg, J. Electrochem. Soc. 124, 1329 (1977).
K. Hirano, K. Inoue and T. Yatsu, J. Photochem. Photobiol. A: Chem. 64, 255 (1992).
O. Ishitani, C. Inoue, Y. Suzuki and T. Ibusuki, J. Photochem. Photobiol. A: Chem. 72, 269 (1993).
T. Mizuno, K. Adhachi, K. Ohta and A. Saji, J. Photochem. Photobiol. A: Chem. 98, 87 (1996).
K. Tennakone, A. H. Jayatissa and S. Punchihewa, J. Photochem. Photobiol. A: Chem. 49, 369 (1989).
I.-H. Tseng, W.-C. Chang and J. C. S. Wu, Appl. Catal. B: Envion. 37, 37 (2002).
S. Kuwabata, H. Uchida, A. Ogawa, S. Hirao and H. Yoneyama, J. Chem. Soc. Chem. Commun., 829 (1995).
M. Anpo, H. Yamashita, Y. Ichihashi and S. Ehara, J. Electroanal. Chem. 396, 21 (1995).
M. Harada, M. Honda, H. Yamashita and M. Anpo, Res. Chem. Intermed. 25, 757 (1999).
S. A. Larson, J. A. Widegren and J. L. Falconer, J. Catal. 157, 611 (1995).
R. I. Bickley and R. K. M. Jayanty, Faraday Discuss. Chem. Soc. 58, 194 (1974).
B. Ohtani, M. Kakimoto, H. Miyadzu, S.-I. Nishimoto and T. Kagiya, J. Phys. Chem. 92, 5773 (1988).
I. Ait-Ichou, M. Formenti, B. Pommier and S. J. Teichner, J. Catal. 91, 293 (1985).
R. I. Bickley, G. Munuera and F. S. Stone, J. Catal. 31, 398 (1973).
H. Yamashita, M. Honda, M. Harada, Y. Ichihashi, M. Anpo, T. Hirao, N. Itoh and N. Iwamoto, J. Phys. Chem. B 102, 10707 (1998).
Y. Ohko, A. Fujishima and K. Hashimoto, J. Phys. Chem. B 102, 1724 (1998).
D. Brinkley and T. Engel, J. Phys. Chem. B 102, 7596 (1998).
J. E. Rekoske and M. A. Barteau, J. Catal. 165, 57 (1997).
J. M. Gallardo-Amores, T. Armaroli, G. Ramis, E. Finocchio and G. Busca, Appl. Catal. B: Environ. 22, 249 (1999).
H. Miyata, K. Hata, T. Nakajima and Y. Kubokawa, Bull. Chem. Soc. Jpn. 53, 2401 (1980).
J. Rabani and M. S. Metheson, J. Am. Chem. Soc. 80, 3175 (1964).
Y. Tabata, in: Pulse Radiolysis, p. 399. CRC Press, Boca Raton, FL (1991).
B. H. J. Bieski, D. E. Cabelli, R. L. Arudi and A. B. Ross, J. Phys. Chem. Ref. Data 14, 1041 (1985).
C. D. Jaeger and A. J. Bard, J. Phys. Chem. 83, 3146 (1979).
J. R. Harbour and M. L. Hair, J. Phys. Chem. 83, 652 (1979).
A. Fujishima and K. Kohayakawa, J. Electrochem. Soc. 122, 1487 (1975).
T. Kawai and T. Sakata, J. Chem. Soc. Chem. Commun, 694 (1980).
A. Mills and S. Le Hunte, J. Photochem. Photobiol. A: Chem. 108, 1 (1997).
G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data 17, 513 (1988).
W. Choi and M. R. Hoffmann, Sci. Technol. 31, 89 (1997).
J. W. T. Spinks and R. J. Woods, in: An Introduction to Radiation Chemistry, 3rd edn, p. 152. Wiley, New York, NY (1990).
A. J. Elliot, Radiat. Phys. Chem. 34, 753 (1989).
K. Hirano, H. Asayama, A. Hoshino and H. Wakatsuki, J. Photochem. Photobiol. A: Chem. 110, 307 (1997).
J. K. Thomas, J. Phys. Chem. 71, 1919 (1967).
E. R. Kantrowitz, M. Z. Hoffman and J. F. Endicott, J. Phys. Chem. 75, 1914 (1971).
A. K. Vijh and B. E. Conway, Chem. Rev. 67, 623 (1967).
B. Kraeutler and A. J. Bard, J. Am. Chem. Soc. 100, 5985 (1978).
B. Kraeutler and A. J. Bard, J. Am. Chem. Soc. 99, 7729 (1977).
B.-J. Liu, T. Torimoto, H. Matsumoto and H. Yoneyama, J. Photochem. Photobiol. A: Chem. 108, 187 (1997).
H. Inoue, H. Moriwaki, K. Maeda and H. Yoneyama, J. Photochem. Photobiol. A: Chem. 86, 191 (1995).
B.-J. Liu, T. Torimoto and H. Yoneyama, J. Photochem. Photobiol. A: Chem. 115, 227 (1998).
H. A. Schwarz and R. W. Dodson, J. Phys. Chem. 93, 409 (1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dey, G.R., Pushpa, K.K. Formation of different products during photo-catalytic reaction on TiO2 suspension in water with and without 2-propanol under diverse ambient conditions. Res Chem Intermed 33, 631–644 (2007). https://doi.org/10.1163/156856707781749883
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856707781749883