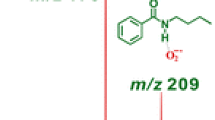
The pulse radiolysis of acidic aqueous solutions of 2-(methylthio)ethanoic acid and S-ethylthioacetone yields acetic acid radicals, ˙CH2COOH, and acetone radicals, ˙CH2-C(O)CH3, respectively. These radicals were identified by time-resolved electron spin resonance. Based on the analogy to previous work on α-(methylthio)acetamide where acetamide radicals were identified in similar situations, the mechanism of the reactions is assigned as bimolecular homolytic substitution (SH2). Support for this assignment is given by density functional theory (DFT) calculations and radical-scavenging experiments with tert-butanol. DFT computations were made on the thermo-chemistry of the SH2 reaction pathway and on the various alternatives to SH2 such as H-abstraction/β-scission.
Similar content being viewed by others
Rights and permissions
About this article
Cite this article
Wisniowski, P., Bobrowski, K., Filipiak, P. et al. Reactions of hydrogen atoms with α-(alkylthio) carbonyl compounds. Time-resolved ESR detection and DFT calculations. Res Chem Intermediat 31, 633–641 (2005). https://doi.org/10.1163/1568567054909023
Published:
Issue Date:
DOI: https://doi.org/10.1163/1568567054909023