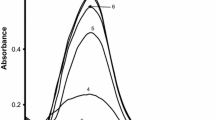
The reactivity of (SCN)˙−2, Br˙−2 and phenoxyl radicals in aqueous solution, generated by pulse radiolysis, was studied on gold and silver nanoparticle surfaces. The spectral changes associated with chemical interaction of radicals with metal nanoparticles were elucidated using time-resolved absorption spectroscopy. The resultant complexes of (SCN)˙−2 and Br˙−2 with Au nanoparticles have shown a strong absorption band at 390 nm and 260 nm, respectively. The stability of the particles after adsorption of SCN−, Br− and phenols was measured by steady-state absorption. It has been shown that the concentration of CTAB plays a significant role in the stabilization of particles in the presence of an adsorbate.
Similar content being viewed by others
Rights and permissions
About this article
Cite this article
Thomas, S., Mahal, H.S., Kapoor, S. et al. Complexation of gold and silver nanoparticles with radiolytically-generated radicals. Res Chem Intermediat 31, 595–603 (2005). https://doi.org/10.1163/1568567054908952
Published:
Issue Date:
DOI: https://doi.org/10.1163/1568567054908952