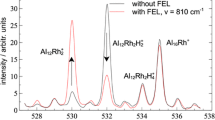
Two examples from the field of physical chemistry are given here in order to demonstrate the versatility of pulse radiolysis. In the study of the early stages in the growth of small silver particles, the trimer silver cluster Ag2+3 exhibits a pronounced position that this cluster represents the changeover from pseudo-first-order kinetics to second-order kinetics. An attempt will be made to describe the structure of this species. The reaction of hydrogen atoms and hydroxyl radicals with aliphatic alcohols is monitored by observing directly the absorption of the resultant radicals. As an application, the reaction H˙ + H2O2 was studied by means of the absorption of alcohol radicals.
Similar content being viewed by others
Rights and permissions
About this article
Cite this article
Janata, E. Pulse radiolysis experiments at the ELBENA facility: on the structure of the trimer silver cluster Ag2+3, and the direct observation of aliphatic alcohol radicals. Res Chem Intermediat 31, 605–611 (2005). https://doi.org/10.1163/1568567054908934
Published:
Issue Date:
DOI: https://doi.org/10.1163/1568567054908934