Abstract
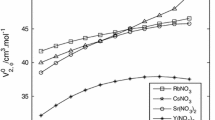
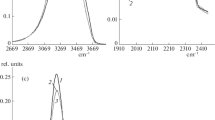
The optical absorption spectra observed by pulse radiolysis of alkaline (NaOH, KOH, RbOH), chloride (LiCl, MgCl2, CaCl2, NaCl, KCl) and perchloride (NaClO4) solutions at temperature 298 K are reported. Some measurements were performed at low temperature with aqueous ionic glasses. With increasing concentration of the above solutes a uniform blue-shift of the maximum of the solvated electron (e¯sol) absorption band is observed. Near infrared (NIR) spectroscopy was so used to examine the properties of water in several concentrated electrolyte solutions. It is shown that some inorganic electrolytes (e.g. NaOH, NaClO4) substantially change the water structure whereas some others (e.g. LiCl, CaCl2) influence water structure insignificantly. The correlation between the ability of excess electron trapping in electrolyte solutions and water structure deduced from NIR spectroscopy is discussed.
Similar content being viewed by others
REFERENCES
M. Anbar and E. J. Hart, J.Phys.Chem. 69, 1244 (1965).
R. J. Woods, B. Lesigne, L. Gilles, C. Ferradini and J. Pucheault, J.Phys.Chem. 79, 2700 (1975).
H. A. Gillis and D. C. Walker, J.Chem.Phys. 65, 4590 (1976).
G. V. Buxton, H. A. Gillis and N. V. Klassen, Can.J.Chem. 54, 367 (1976).
H. A. Gillis, G. G. Teather and G. V. Buxton, Can.J.Chem. 56, 1889 (1978).
T. Q. Nguyen, D. C. Walker and H. A. Gillis, J.Chem.Phys. 69, 1038 (1978).
G. Dolivo and L. Kevan, J.Chem.Phys. 70, 2599 (1979).
G. Dolivo and L. Kevan, J.Chem.Phys. 70, 5489 (1979).
S. A. Rice, G. Dolivo and L. Kevan, J.Chem.Phys. 70, 18 (1979).
J. W. van Leeuwen, L. H. Straver and H. Nauta, J.Phys.Chem. 83, 3008 (1979).
N. V. Klassen, R. J. Adams, G. G. Teather and C. K. Ross, J.Phys.Chem. 84, 3609 (1980).
J. W. van Leeuwen, M. G. J. Heijman and H. Nauta, Radiat.Phys.Chem. 17, 367 (1981).
G. V. Buxton, M. Medley, G. A. Salmon, P. J. Baugh, R. Catterall and P. B. Williams, J.Chem.Soc.Faraday Trans.I 81, 3067 (1985).
I. V. Kreitus, J.Phys.Chem. 89, 1987 (1985).
H. Hase, C. Hosokawa and T. Higashimura, Radiat.Phys.Chem. 29, 209 (1987).
H. Hase and T. Higashimura, Radiat.Phys.Chem. 30, 157 (1987).
A. V. Veselov and R. W. Fessenden, J.Phys.Chem. 97, 3497 (1993).
J. Kroh, S. Noda, K. Yoshida and H. Yoshida, Bull.Chem.Soc.Japan 51, 1961 (1978).
J. Kroh and P. Polevoi, Radiat.Phys.Chem. 11, 111 (1978).
Y. Kondo, M. Aikawa, T. Sumiyoshi, M. Katayama and J. Kroh, J.Phys.Chem. 84, 2544 (1980).
Cz. Stradowski and J. Kroh, Radiat.Phys.Chem. 15, 349 (1980).
M. Wolszczak and Cz. Stradowski, Radiat.Phys.Chem. 23, 403 (1984).
M. Wypych, Z. Czerwik and J. Kroh, Radiat.Phys.Chem. 33, 145 (1989).
M. Wypych and J. Kroh, Radiat.Phys.Chem. 36, 127 (1990).
M. Wypych and J. Kroh, Bull.Pol.Ac.: Chem. 40, 155 (1992).
M. Wypych, in: Properties and Reactions of Radiation Induced Transients.Selected Topics, J. Mayer (Ed.), p. 53. Polish Scientific Publishers PWN, Warszawa (1999).
Y. Gauduel, in: Ultrafast Dynamics of Chemical Systems, J. D. Simon (Ed.), p. 81. Kluwer Academic Publishers, Dordrecht (1994).
Y. Gauduel, J.Mol.Liq. 63, 1 (1995).
A. N. Asaad, N. Chandrasekhar, A. W. Nashed and P. Krebs, J.Phys.Chem.A 103, 6339 (1999).
W. M. Bartczak, M. Hilczer and J. Kroh, Radiat.Phys.Chem. 17, 431 (1981).
S. Karolczak, K. Hodyr and M. Polowinski, Radiat.Phys.Chem. 39, 1 (1992).
F. Franks (Ed.), in: Water: A Comprehensive Treatise. Plenum Press, New York (1973).
G. C. Pimentel and A. L. McClellan, in: The Hydrogen Bond, p. 83. W. H. Freeman, San Francisco (1990).
E. J. Heilweil, Science 283, 1467 (1999).
C. A. Angell and V. Rodgers, J.Chem.Phys. 80, 6245 (1984).
V. S. Langford, A. J. McKinley and T. I. Quickenden, J.Phys.Chem.A 105, 8916 (2001).
W. M. Grundy and B. Schmitt, J.Geophys.Res.E 103, 25809 (1998).
R. C. Dougherty and L. N. Howard, J.Chem.Phys. 109, 7379 (1998).
Y. Maeda and H. Kitano, Spectrochim.Acta A 51, 2433 (1995).
D. J. Barnes, in: Ionomers.Characterization, Theory, and Applications, S. Schlick (Ed.), Chapter 6. CRC Press, Boca Raton (1996).
W. A. P. Luck, D. Schiöberg and U. Siemann, J.Chem.Soc.Faraday II 76, 136 (1980).
Z. Kecki, P. Dryjanski and E. Kozlowska, Rocz.Chem. 42, 1749 (1968).
W. C. McCabe, S. Subramanian and H. F. Fisher, J.Phys.Chem. 74, 4360 (1970).
V. J. Frost and K. Molt, J.Mol.Struct. 410/411, 573 (1997).
A. A. Zavitsas, J.Phys.Chem.B 105, 7805 (2001).
H. G. Hertz, Angew.Chem. 82, 91 (1970).
I. C. Baianu, N. Boden, D. Lightowlers and M. Mortimer, Chem.Phys.Letters 54, 169 (1978).
J. R. Scherer, in: Advances in Infrared and Raman Spectroscopy, Volume 5, R. J. H. Clark R. E. Hester (Eds), Chapter 3. Heyden, London (1978).
W. A. P. Luck, Angew.Chem. 92, 29 (1980).
X. Zhou, P. Hines and M. W. Borer, J.Pharm.Biomed.Anal. 17, 219 (1998).
T. Q. Nguyen and D. C. Walker, J.Chem.Soc.Faraday Trans.I 73, 1958 (1977).
F.-Y. Jou and G. R. Freeman, J.Phys.Chem. 83, 2383 (1979).
Rights and permissions
About this article
Cite this article
Wolszczak, M., Wypych, M., Tomczyk, M. et al. Water structure and electron trapping in aqueous ionic solutions. Research on Chemical Intermediates 28, 537–549 (2002). https://doi.org/10.1163/15685670260373308
Issue Date:
DOI: https://doi.org/10.1163/15685670260373308