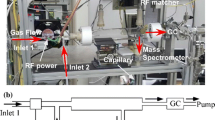
Abstract
Methane is converted to C2 products in a microwave plasma under pressure up to 400 torr at maximum plasma power of 100 W. Steam is introduced with methane into the plasma zone in order to suppress coke formation. Major products are C2 hydrocarbons. Small amounts of benzene are also formed. Very small amounts of some unusual highly unsaturated hydrocarbons are also formed. Oxygenated products are CO and CO2. The conversion and yields are related to experimental variables by an empirical second order linear model. The conversion of methane ranges from 10 to 60%. The yield of C2 products ranges from 5 to 68%. The major C2 product is acetylene.
Similar content being viewed by others
REFERENCES
R. G. Mallinson, C. M. Slipcevich and S. Ruseh, Reprints of the 194th National Meeting of the ACS, Section of the Fuel Chemistry, New Orleans, Louisiana, 32 (3), 226 (1987).
S. S. Shepelev, H. D. Gesser and N. R. Hunter, Plasma Chem. Plasma Phys. 13, 479 (1993).
S. L. Suib and Z. Zhang, US Patent, No. vn5,015,349 (May 14, 1991).
S. L. Suib and Z. Zhang, US Patent, No. vn5,131,993 (July 21, 1992).
R. P. Zerger, S. L. Suib and Z. Zhang, in: Symposium on Natural Gas Upgrading II, Preprint ACS, Div. Pet. Chem., San Francisco, pp. 344-348 (1992).
. J. Huang and S. L. Suib, J. Phys. Chem. 97, 9405 (1993).
R. J. McCarthy, J. Chem. Phys. 22, 1360 (1954).
J. E. Flinn (Ed.), Engineering, Chemistry and Use of Plasma Reactors, Chem. Eng. Prog. Sym. Ser., Vol. 112 (1971).
T. Junl-Dam and N. F. Brockmeier, Ind. Eng. Chem. Prod. Res. Dev. 9, 388 (1970).
H. V. Boeing, Fundamentals of Plasma Chemistry and Technology. Technomic, Lancaster, UK (1988).
R. F. Baddour and R. S. Timmius (Eds), The Application of Plasmas to Chemical Processing. MIT Press, Cambridge, MA (1967).
J. Huang, M. V. Badani, S. L. Suib, J. B. Harrison and M. Kablauoi, J. Phys. Chem. 98, 206 (1994).
W. Y. Lui, in: 7th International Plasma Chemistry Conference (1985).
R. Mach and H. Drost, in: 6th International Symposium on Plasma Chemistry, Montreal, Quebec, Canada (1983).
Y. Kawahara, J. Phys. Chem. 73, 1648 (1969).
H. Wiener and M. Burton, J. Amer. Chem. Soc. 75, 5815 (1953).
Y. Kawahara, US Patent, No. vn3,663.394 (May 16, 1972).
W. J. Murphy and A. Ravella, Eur. Patent, 0435591A2 (Dec 20, 1990).
DOE/ CE/ 15459-T2, April 23, 1991.
C. M. Shaughnessy, An analysis of the microwave plasma as a chemical reactor, PhD Thesis, The University of Texas at Austin (1971).
K. G. Micllewiz, J. J. Urh and J. W. Carnahan, Spectrochim Acta 40B, 493 (1985).
C. I. M. Beenakker, Spectrochim. Acta 31B, 483 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huang, J., Suib, S., Harrison, J.B. et al. Activation of methane in microwave plasmas at high pressure. Research on Chemical Intermediates 27, 643–658 (2001). https://doi.org/10.1163/156856701317051743
Issue Date:
DOI: https://doi.org/10.1163/156856701317051743