Abstract
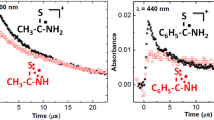
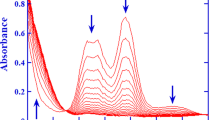
The reactions of e− aq, H-atoms, OH radicals and some one electron oxidants and reductants were studied with dithio-oxamide (DTO) in aqueous solutions using pulse radiolysis technique. The transient species formed by the reaction of e− aq with DTO at pH 6.8 has an absorption band with λ max at 380 nm and is reducing in nature. H-atom reaction with DTO at pH 6.8 also produced the same transient species. The semi-reduced species was found to be neutral indicating that the electron adduct gets protonated quickly. However at pH 1, the species produced by H-atom reaction had a different spectrum with λ max at 360 and 520 nm. Reaction of acetone ketyl radicals and CO2 − radicals with DTO at pH 6.8 gave transient spectra which were identical to that obtained by e− aq reaction. However at pH 1, the spectrum obtained by the reaction of acetone ketyl radicals with DTO was similar to that obtained by H-atom reaction at that pH. The transient species formed by OH radical reaction with DTO in the pH range 1–9.2 also has two absorption maxima at 360 and 520 nm. This spectrum was identical with the spectrum obtained by H-atom reaction at pH 1. This means that all these radicals viz. OH, H-atom and (CH3)2COH radicals react with DTO at pH 1 by H-abstraction mechanism. The transient species produced was found to be sensitive to the presence of oxygen. One-electron oxidizing radicals such as Br2 −· and SO4 −· radicals reacted with DTO at neutral pH to give the same species as produced by OH radical reaction having absorption maxima at 360 to 520 nm. At acidic pHs, only Br2 −· and Cl2 −· radicals were able to oxidize DTO to give the same species as produced by OH radical reaction. The semioxidized species is a resonance stabilized species with the electron delocalized over the-N-C-S bond. This species was found to be neutral and non-oxidizing in nature.
Similar content being viewed by others
References
G.R. Dey, D.B. Naik, K. Kishore, and P.N. Moorthy, Radiat. Phys. Chem. 43, 365 (1994).
G.R. Dey, D.B. Naik, K, Kishore, and P.N. Moorthy, J. Chem. Soc. Perkin Trans. 2 1625 (1994).
G.R. Dey, D.B. Naik, K. Kishore, and P.N. Moorthy, Res. Chem. Intermed. 21, 47 (1995).
K. Kishore, G.R. Dey, and P.N. Moorthy, J. Phys. Chem. 99, 13476 (1995).
K. Kishore, P. Dwibedy, G.R. Dey, and P.N. Moorthy, Res. Chem. Intermed. 24, 35 (1998).
G.R. Dey, P. Dwibedy, D.B. Naik, K. Kishore, and P.N. Moorthy, Res. Chem. Intermed. 25, 403 (1999).
S.N. Guha, P.N. Moorthy, K. Kishore, D.B. Naik, and K.N. Rao, Proc. Indian Acad. Sci. (Chem. Sci.) 99, 261 (1987).
E.M. Fielden, Chemical Dosimetry of Pulsed Electron and X-ray Source in the 1–20 MeV in the Study of Fast Processes and Transient Species by Electron Pulse Radiolysis, J.H. Baxendale and F. Busi (Eds.), D. Reidel, Dordrecht, 1984, p. 49.
M.S. Panajkar, P.N. Moorthy, and N.D. Shirke, BARC Report, BARC-1470, 1989.
G.V. Buxton, C.L. Greenstock, W.P. Helman, and A.B. Ross, J. Phys. Chem. Ref. Data 17, 513 (1988).
A.J. Swallow, The Study of Fast Processes and Transient Species by Electron Pulse Radiolysis, J.H. Baxendale and F. Busi (Eds.), Reidel Publishing Co., London, 1982, p. 297.
P. Wardman, J. Phys. Chem. Ref. Data 18, 1637 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dey, G.R., Naik, D.B., Dwibedy, P. et al. Pulse radiolysis study of dithio-oxamide in aqueous solutions. Res Chem Intermed 26, 309–318 (2000). https://doi.org/10.1163/156856700X00264
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856700X00264