Abstract
Lack of insulin results in a catabolic state in subjects with insulin-dependent diabetes mellitus which is reversed by insulin treatment. Amino acid supply, especially branched chain amino acids such as leucine, enhances protein synthesis in both animal and human studies. This small study was undertaken to assess the acute effect of supplemental leucine on protein metabolism in adolescents with type 1 diabetes. L-[1-13C] Leucine was used to assess whole-body protein metabolism in six adolescent females (16–18 yrs) with type 1 diabetes during consumption of a basal diet (containing 58 μ moles leucine/kg/h) and the basal diet with supplemental leucine (232 μ moles leucine/kg/h). Net leucine balance was significantly higher with supplemental leucine ( μ moles leucine/kg body weight/hr) than with the basal diet (
μ moles leucine/kg body weight/hr) than with the basal diet ( ,
,  ) due to reduced protein degradation (
) due to reduced protein degradation ( μ moles leucine/kg body weight/hr) compared to the basal diet (
μ moles leucine/kg body weight/hr) compared to the basal diet ( ,
,  ).
).
Similar content being viewed by others
1. Introduction
Whole body protein catabolism results when the rate at which body protein is synthesized is less than the rate at which body protein is degraded. Insulin is among the many factors which regulate body protein metabolism. The lack of insulin associated with type 1 diabetes results in the loss of body protein, and insulin treatment reverses the loss of body protein resulting in increased lean body mass [1]. Several animal studies have demonstrated the importance of insulin in regulating the rate of protein synthesis particularly in muscle tissue (e.g., [2–7]). In contrast, studies of whole-body protein metabolism in adult humans with type 1 diabetes [8–13] and prepubertal children [14] have suggested that the primary effect of insulin is a reduction in the rate of protein degradation rather than a stimulation of the rate of protein synthesis. While provision of insulin to individuals with type 1 diabetes enhances the accretion of body protein mass, there are also data from adults [15] and adolescents females [16] suggesting that long-term insulin therapy also results in accumulation of body fat compared to nondiabetic individuals. The current study was undertaken to determine if the anabolic effect of insulin on protein metabolism could be enhanced by the concurrent provision of a diet enriched with the amino acid, leucine.
In clinical studies, solutions of branched-chain amino acids have improved nitrogen balance in patients with surgical stress [17–19]. In patients with liver carcinoma, provision of branched-chain amino acids has been demonstrated to result in decreased morbidity and improved quality-of-life [20], reduced complications following surgery [21], and decreased length of hospital stay [22]. In healthy subjects, provision of essential amino acids in both young and old subjects results in a stimulation of the rate at which muscle protein is synthesized [23, 24]. In elderly subjects, enriching the essential amino acid supplement with additional leucine results in a further stimulation in the rate of muscle protein synthesis in elderly, but not young subjects [25]. Even in the absence of other essential amino acids, leucine supplementation has been demonstrated to be sufficient to enhance the rate of muscle protein synthesis in elderly subjects [26]. In animal studies, leucine alone stimulated the rate of muscle protein synthesis, although the plasma concentrations of insulin were also increased when leucine was given [27]. When the increase in insulin secretion was prevented, the ability of leucine to stimulate the rate of muscle protein synthesis was also blocked [27] suggesting that the change in protein synthesis was insulin dependent. In contrast, in another study, leucine has been shown to stimulate the rate of muscle protein synthesis in diabetic animals suggesting that there is also an insulin-independent mechanism for the ability of leucine to stimulate muscle protein synthesis [28].
The current study was undertaken to determine if adolescents with type 1 diabetes respond to leucine supplementation of a complete diet with an increase in protein retention. If leucine were able to increase net protein retention, then there may be a role for increasing the leucine content of the diet for adolescents with type 1 diabetes.
2. Methods
2.1. Protocol
The study was conducted in 6 adolescent females with type 1 diabetes (Table 1). The subjects were studied over a 48-hour period at the General Clinical Research Center of Stony Brook University Medical Center. Subjects were admitted the evening preceding two sequential study days. The protocol was approved by the Committee of Research Involving Human Subjects, the Institutional Review Board. Written consent from the parents of minors and written consent or assent from the subjects was obtained after explaining the purpose, nature, and potential risks of the study. A urine pregnancy test was done on all subjects to exclude pregnancy.
The two study days consisted of six-hour periods beginning at 7 AM after an overnight fast. The two study days were consecutive to minimize any impact of menstrual cycle on protein metabolism. During the study periods, the subjects consumed hourly meals of Carnation Ready-to-Eat Instant Breakfast with 19% of calories from fat, 57% of calories from carbohydrate, and 22% from protein. The hourly meals contained 1/12 of the individual subject's daily caloric requirement (resting energy expenditure from [29] ×1.6). Subjects randomly consumed the basal diet on one study day and the basal diet with added L-leucine (Ajinomoto, Inc, Raleigh, NC) on the other study day. Leucine intake from the basal diet was 58 μ moles/kg/h (or 7 mg/kg/h) and with added leucine the dietary intake of leucine was 232 μ moles (30 mg)/kg/h. All subjects discontinued their subcutaneous insulin infusion via insulin pump on the morning of study and were maintained on an insulin drip with a bolus of 0.05–0.1 units/kg and 0.025–0.15 units/kg/hr to maintain plasma glucose between 80–180 mg/dL. Plasma glucose was monitored every 15 minutes and the rate of insulin infusion adjusted to maintain plasma glucose in the desired range (Beckman II Glucose Analyzer).
L-[1-13C] leucine (Cambridge Isotopes, Andover, MA) was infused intravenously to assess whole-body protein kinetics as described previously for example [30, 31]. Labeled leucine was given as a bolus of 6 μ moles per kg body weight followed by a continuous infusion of 6 μ moles per kg body weight per hour through an in-dwelling cannula in a forearm vein. A heated vein of the contralateral hand was cannulated and used for blood sampling. Samples were collected hourly and then every 15 minutes in the last hour for the assessment of plasma glucose, insulin, amino acids, and the enrichment of 13C-ketoisocaproate (KIC) and L-[1-13C] leucine. Samples of expired air were collected at the same time as the blood samples for the assessment of enrichment of 13CO2. The rate of CO2 production was measured with a ventilated hood system (Delta Trac Respiratory Gas Monitor, Yorba Linda, CA) during the last hour of each study day.
2.2. Protein Kinetics
The enrichment of L-[1-13C] leucine and [13C]KIC in plasma was measured as described previously, for example, [30–32] with a VG MD 800 quadrupole gas chromatography mass spectrometry (Fisons MD 800, San Jose, CA). The enrichment of L-[1-13C] leucine was measured after conversion to the tertiary butyldimethylsilyl- and [13C]KIC after conversion to the quinoxalinol tertiary butyldimethylsilyl-derivative. The enrichment of breath 13CO2 was measured by gas isotope ratio-mass spectrometry at the General Clinical Research Center at the University of Vermont with a VG SIRA II Isotope Ratio Mass Spectrometer (Middlewich, Cheshire, UK) as described previously [33].
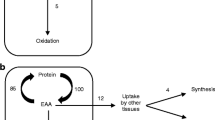
The enrichment of plasma KIC was used as a surrogate for intracellular leucine enrichment [34]. The assessment of protein metabolism was made from the enrichment of [13C]KIC and 13CO2 during the last hour of the 6 hours of feeding. Endogenous leucine flux ( ) was calculated from rate (
) was calculated from rate ( ) and the enrichment (
) and the enrichment ( ) of leucine infused and the enrichment of plasma KIC (
) of leucine infused and the enrichment of plasma KIC ( ) with the formula below and corrected for the amount of leucine infused.
) with the formula below and corrected for the amount of leucine infused.

The rate of leucine oxidation was derived from the rate of expired 13CO2 production (breath enrichment of 13CO2 × CO2 production) with correction for incomplete recovery of CO2 [31]. The rate of protein degradation, in μ mole leucine/kg/hr, was calculated as the rate of flux minus the rate of leucine intake, and the rate of protein synthesis was calculated as flux minus oxidation [35]. Net protein balance was calculated from the difference between the rate of protein degradation and the rate of protein synthesis.
2.3. Plasma Insulin and Glucose
The concentration of glucose in plasma was determined with a glucose oxidase method carried out with a Beckman II Glucose Analyzer. Plasma insulin concentrations were determined by radioimmunoassay (Diagnostics Products Corp, Los Angles, CA).
2.4. Biostatistics
All data are expressed as mean ± SEM. Statistical analysis was performed with a two-tailed paired  -test for comparison of the data between the periods with basal diet and with basal diet and supplemental leucine. Differences with
-test for comparison of the data between the periods with basal diet and with basal diet and supplemental leucine. Differences with  were considered statistically significant.
were considered statistically significant.
3. Results
The study was conducted in 6 female subjects with a mean age of  years who had type 1 diabetes for a duration of
years who had type 1 diabetes for a duration of  years. (Table 1). Prior to enrollment in the study, all subjects received insulin through continuous subcutaneous infusion sets and had variable control of their disease with hemoglobin A1C values ranging from 7.1–10.5%.
years. (Table 1). Prior to enrollment in the study, all subjects received insulin through continuous subcutaneous infusion sets and had variable control of their disease with hemoglobin A1C values ranging from 7.1–10.5%.
During the two study days, conducted in random order, subjects received an intravenous infusion of insulin to maintain plasma glucose. There was no significant difference in the plasma glucose levels on the day with basal diet ( mg/dL) compared with the day with the basal diet plus supplemental leucine (
mg/dL) compared with the day with the basal diet plus supplemental leucine ( ), as shown in Figure 1. However, the amount of insulin required to maintain these levels of plasma glucose was significantly different on the two study days, with greater amounts of insulin required on the study day with supplemental leucine (
), as shown in Figure 1. However, the amount of insulin required to maintain these levels of plasma glucose was significantly different on the two study days, with greater amounts of insulin required on the study day with supplemental leucine ( units) compared with the day when the basal diet was consumed (
units) compared with the day when the basal diet was consumed ( units,
units,  ) and higher levels of plasma insulin (Figure 2).
) and higher levels of plasma insulin (Figure 2).
Plasma glucose concentrations in type 1 diabetic adolescent girls consuming a basal diet or a diet supplemented with L-leucine. Plasma concentrations of glucose (mg/dl) during consumption of the basal diet (19% of calories from fat, 57% of calories from carbohydrate, and 22% from protein) or the basal diet with supplemental L-leucine (total leucine content of 4 times the basal diet).
Plasma insulin concentrations in type 1 diabetic adolescent girls consuming a basal diet or a diet supplemented with L-leucine. Plasma concentrations of insulin (mU/L) during consumption of the basal diet (19% of calories from fat, 57% of calories from carbohydrate, and 22% from protein) or the basal diet with supplemental L-leucine (total leucine content of 4 times the basal diet).
In response to the consumption of the basal diet with supplemental leucine, the concentration of leucine in plasma was significantly increased compared to the concentration during consumption of the basal diet alone (Table 2). There was a significant decrease in the plasma concentration of the other branched-chain amino acids, isoleucine, and valine, as well as asparagine, histidine, threonine, and tyrosine.
Provision of supplemental leucine in the diet significantly reduced the enrichment of plasma leucine ( moles percent excess) compared to the enrichment of leucine during the consumption of the basal diet (
moles percent excess) compared to the enrichment of leucine during the consumption of the basal diet ( ,
,  ). The enrichment of plasma KIC was also significantly different between the 2 days (
). The enrichment of plasma KIC was also significantly different between the 2 days ( with supplemental leucine and
with supplemental leucine and  with the basal diet,
with the basal diet,  ). All parameters of whole body protein metabolism (shown in Figure 3) were calculated from the enrichment of KIC in the plasma. The rate at which whole-body protein was synthesized did not differ between the study day when subjects consumed the basal diet and the study day when the diet was supplemented with additional leucine equivalent four times the amount in the basal diet (
). All parameters of whole body protein metabolism (shown in Figure 3) were calculated from the enrichment of KIC in the plasma. The rate at which whole-body protein was synthesized did not differ between the study day when subjects consumed the basal diet and the study day when the diet was supplemented with additional leucine equivalent four times the amount in the basal diet ( μ moles leucine/kg body weight/hr with supplemental leucine compared to
μ moles leucine/kg body weight/hr with supplemental leucine compared to  with the basal diet). In contrast, the rate at which whole-body protein was degraded was significantly reduced when the diet was enriched with leucine (
with the basal diet). In contrast, the rate at which whole-body protein was degraded was significantly reduced when the diet was enriched with leucine ( versus
versus  μ moles leucine/kg body weight/hr,
μ moles leucine/kg body weight/hr,  ). The rate of leucine oxidation was significantly higher when additional leucine was added to the diet as is to be expected (
). The rate of leucine oxidation was significantly higher when additional leucine was added to the diet as is to be expected ( versus
versus  ,
,  ). Protein balance or the net accretion of body protein, that is, the rate at which protein was synthesized relative to the rate at which protein was degraded was significantly improved with the addition of supplemental leucine to the basal diet (
). Protein balance or the net accretion of body protein, that is, the rate at which protein was synthesized relative to the rate at which protein was degraded was significantly improved with the addition of supplemental leucine to the basal diet ( versus
versus  ,
,  ).
).
Leucine kinetics in type 1 diabetic adolescent girls consuming a basal diet or a diet supplemented with L-leucine. Protein synthesis, protein degradation, leucine oxidation, and protein balance were calculated from the enrichment of plasma [13C]KIC, 13CO2, and CO2 production as described in Materials and Methods. The data are expressed as μ moles of leucine/kg body weight/h during consumption of the basal diet (19% of calories from fat, 57% of calories from carbohydrate, and 22% from protein) or the basal diet with supplemental L-leucine (total leucine content of 4 times the basal diet). Differences between the study day with the basal diet and the study day with the basal diet supplemented with L-leucine were statistically significant, * .
.
4. Discussion
This study was undertaken to determine if supplemental leucine could maximize the utilization of dietary protein in type 1 diabetic adolescents. If an anabolic effect were observed, the study also had the potential to provide information about the importance of insulin in mediating the effect of leucine in the human context, since these subjects were unable to increase circulating insulin concentrations in response to supplemental dietary leucine. This acute study, conducted over two study days of 6 hours each, suggests that supplemental dietary leucine equivalent to four times the leucine content of the basal diet may improve the net accumulation of whole-body protein through a decrease in the rate at which protein is degraded. The study design to examine the acute response (between 5-6 h) to supplemental dietary leucine was chosen so that the results would be somewhat comparable to animal studies (e.g., [2, 27, 28, 36–38]) examining this same phenomenon. Sequential study days were used to compare the responses to the basal diet with responses to the leucine-supplemented diet: a study design which minimized potentially confounding variables such as stage of menstrual cycle or alterations in exercise regimen between study days. Since the acute effects on protein metabolism associated with feeding are unlikely to extend through the overnight fasting period into the following day, it is unlikely that there was a carry-over effect on the second study day from the diet consumed on the first study day. However, to avoid such a potential, study days were randomized with respect to the order of leucine-supplemented or basal diet as the first study day.
Both insulin and dietary protein (i.e., amino acids) have an anabolic effect on protein metabolism, but animal studies have suggested that leucine alone also has the ability to augment the anabolic effect of insulin on the synthesis of muscle protein (e.g., [2, 27]). Further studies have suggested that leucine has an anabolic effect on muscle protein synthesis in diabetic animals suggesting that at least a part of the leucine effect is independent of insulin [28]. In animal studies, the insulin-mediated effect of leucine on protein synthesis is mediated through a mechanism involving the regulation of mRNA translation initiation [27, 36–38] while the mechanism of the insulin-independent effect is not known.
Studies in adults with type 1 diabetes have suggested that the anabolic effect of insulin on whole-body protein metabolism, particularly protein synthesis, is influenced by the supply of amino acids as well as insulin (e.g., [8, 39]). In healthy subjects, a complete mixture of amino acids is not required; just the essential amino acids are sufficient to stimulate the rate of protein synthesis in muscle tissue [23, 24]. Studies in elderly subjects suggest that leucine specifically plays an important role in the regulation of the rate of synthesis of muscle tissue [25, 26]. The current study in type 1 adolescents also suggests an anabolic effect of leucine on protein metabolism, but with more of an impact on the rate at which protein is degraded rather than the rate at which protein is synthesized. However, the present study measured whole-body rather than the specific effects in muscle tissue.
Although it was anticipated that the leucine would have the ability to stimulate protein synthesis in subjects with insulin-dependent diabetes in line with the studies from diabetic animals [28], this was not the case. Rather a heretofore unreported effect of supplemental dietary leucine to induce hyperglycemia compared to the glucose concentrations which were observed with the consumption of the basal diet without additional leucine. Experimentally, since these subjects are unable to increase insulin secretion to offset the increase in the concentration of plasma glucose, an increase in the infusion of exogenous insulin was necessary to maintain plasma glucose concentrations at the desired levels. Since nutritional supplements like leucine are available over-the-counter and touted as anabolic agents, the potential for supplemental leucine to affect not only protein metabolism but also glucose metabolism is clinically important. This effect of leucine to increase the concentration of plasma glucose in human subjects is somewhat surprising given animal studies reporting that leucine increases tissue sensitivity to insulin, at least in muscle tissue (e.g., [2, 27, 36–38]).
There are a number of limitations to the present study. Firstly, this study measured the responses of whole-body protein metabolism rather than the specific effect of leucine on muscle tissue. However, a study examining muscle protein synthesis from biopsy specimens would not be ethical in this adolescent population. Secondly, the study examined only six subjects. Nevertheless, the observed effect of dietary supplementation with leucine on net protein balance was quite large. A retrospective power calculation of the number of subjects needed to show an effect of this size and variability with 80% power and a two-sided significance level of 0.05 is only 3, suggesting validity of the conclusion despite the small number of subjects. A third limitation of the study is the variability in glycemic control of the subjects. Although it is not possible to determine with certainty that long-term glycemic control does not affect the acute response of protein metabolism to dietary intake, the data suggest that this is not the case. The improvement in protein balance with leucine supplementation was similar (66.8 μ moles of leucine/kg body weight/h) to that of the three subjects with poorer glycemic control (77.1 μ moles of leucine/kg body weight/h in subjects with HdA1c 8.9–10.5). In conclusion, this acute study of 6 hours of feeding supplemented with leucine suggests a positive role for whole-body protein balance. Now it is important to ascertain if longer-term studies with supplemental dietary leucine can provide improvement in lean body mass. However, these longer-term studies must also determine the effect of prolonged leucine administration on the concentration of plasma glucose and whether or not there is a need to increase exogenous insulin with leucine administration.
Abbreviations
- KIC:
-
 -ketoisocaproate.
-ketoisocaproate.
References
Walsh CH, Soler NG, James H: Studies in whole body potassium and whole body nitrogen in newly diagnosed diabetics. Quarterly Journal of Medicine. 1976, 45 (178): 295-301.
Baillie AGS, Garlick PJ: Responses of protein synthesis in different skeletal muscles to fasting and insulin in rats. The American Journal of Physiology. 1991, 260 (6): E891-E896.
Flaim KE, Copenhaver ME, Jefferson LS: Effects of diabetes on protein synthesis in fast- and slow-twitch rat skeletal muscle. The American Journal of Physiology. 1980, 2 (1): E88-E95.
Garlick PJ, Fern M, Preedy VR: The effect of insulin infusion and food intake on muscle protein synthesis in postabsorptive rats. Biochemical Journal. 1983, 210 (3): 669-676.
Jefferson LS: Role of insulin in the regulation of protein synthesis. Diabetes. 1980, 29 (6): 487-496.
Jefferson LS, Li JB, Rannels SR: Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. Journal of Biological Chemistry. 1977, 252 (4): 1476-1483.
Pain VM, Albertse EC, Garlick PJ: Protein metabolism in skeletal muscle, diaphragm, and heart of diabetic rats. The American Journal of Physiology. 1983, 245 (6): E604-E610.
Luzi L, Castellino P, Simonson DC, Petrides AS, DeFronzo RA: Leucine metabolism in IDDM. Role of insulin and substrate availability. Diabetes. 1990, 39 (1): 38-48. 10.2337/diabetes.39.1.38.
Nair KS, Ford GC, Halliday D: Effect of intravenous insulin treatment on in vivo whole body leucine kinetics and oxygen consumption in insulin-deprived type I diabetic patients. Metabolism: Clinical and Experimental. 1987, 36 (5): 491-495. 10.1016/0026-0495(87)90049-7.
Pacy PJ, Bannister PA, Halliday D: Influence of insulin on leucine kinetics in the whole body and across the forearm in post-absorptive insulin dependent diabetic (type 1) patients. Diabetes Research. 1991, 18 (4): 155-162.
Pacy PJ, Nair KS, Ford C, Halliday D: Failure of insulin infusion to stimulate fractional muscle protein synthesis in type I diabetic patients. Anabolic effect of insulin and decreased proteolysis. Diabetes. 1989, 38 (5): 618-624. 10.2337/diabetes.38.5.618.
Robert JJ, Beaufrere B, Koziet J: Whole body de novo amino acid synthesis in type I (insulin-dependent) diabetes studied with stable isotope-labeled leucine, alanine, and glycine. Diabetes. 1985, 34 (1): 67-73. 10.2337/diabetes.34.1.67.
Tessari P, Biolo G, Inchiostro S, Sacca L, Nosadini R, Boscarato MT, Trevisan R, Vigili De Kreutzenberg S, Tiengo A: Effects of insulin on whole body and forearm leucine and KIC metabolism in type 1 diabetes. The American Journal of Physiology. 1990, 259 (1): E96-E103.
Vogiatzi MG, Nair KS, Beckett PR, Copeland KC: Insulin does not stimulate protein synthesis acutely in prepubertal children with insulin-dependent diabetes mellitus. Journal of Clinical Endocrinology and Metabolism. 1997, 82 (12): 4083-4087. 10.1210/jc.82.12.4083.
Carlson MG, Campbell PJ: Intensive insulin therapy and weight gain in IDDM. Diabetes. 1993, 42 (12): 1700-1707. 10.2337/diabetes.42.12.1700.
Ingberg CM, Särnblad S, Palmér M, Schvarcz E, Berne C, Åman J: Body composition in adolescent girls with type 1 diabetes. Diabetic Medicine. 2003, 20 (12): 1005-1011. 10.1046/j.1464-5491.2003.01055.x.
Cerra F, Blackburn G, Hirsch J: The effect of stress level, amino acid formula, and nitrogen dose on nitrogen retention in traumatic and septic stress. Annals of Surgery. 1987, 205 (3): 282-287. 10.1097/00000658-198703000-00011.
Cerra FB, Mazuski J, Teasley K: Nitrogen retention in critically ill patients is proportional to the branched chain amino acid load. Critical Care Medicine. 1983, 11 (10): 775-778. 10.1097/00003246-198310000-00003.
Freund H, Hoover HC, Atamian S, Fischer JE: Infusion of the branched chain amino acids in postoperative patients. Anticatabolic properties. Annals of Surgery. 1979, 190 (1): 18-23. 10.1097/00000658-197907000-00004.
Poon RTP, Yu WC, Fan ST, Wong J: Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Alimentary Pharmacology and Therapeutics. 2004, 19 (7): 779-788. 10.1111/j.1365-2036.2004.01920.x.
Fan ST, Lo CM, Lai ECS, Chu KM, Liu CL, Wong J: Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. New England Journal of Medicine. 1994, 331 (23): 1547-1552. 10.1056/NEJM199412083312303.
Meng WCS, Leung KL, Ho RLK, Leung TWT, Lau WY: Prospective randomized control study on the effect of branched-chain amino acids in patients with liver resection for hepatocellular carcinoma. Australian and New Zealand Journal of Surgery. 1999, 69 (11): 811-815. 10.1046/j.1440-1622.1999.01701.x.
Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR: Amino acid ingestion improves muscle protein synthesis in the young and elderly. The American Journal of Physiology. 2004, 286 (3): E321-E328.
Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR: Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. American Journal of Clinical Nutrition. 2003, 78 (2): 250-258.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR: A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. The American Journal of Physiology. 2006, 291 (2): E381-E387.
Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D: Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. Journal of Physiology. 2006, 575 (1): 305-315. 10.1113/jphysiol.2006.110742.
Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS: Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. The American Journal of Physiology. 2002, 282 (5): E1092-E1101.
Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, Jefferson LS: Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002, 51 (4): 928-936. 10.2337/diabetes.51.4.928.
Institute of Medicine (U.S.). Panel on Macronutrients , Institute of Medicine (U.S.). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes : Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. 2005, National Academies Press, Washington, DC, USA
Godil MA, Wilson TA, Garlick PJ, McNurlan MA: Effect of insulin with concurrent amino acid infusion on protein metabolism in rapidly growing pubertal children with type 1 diabetes. Pediatric Research. 2005, 58 (2): 229-234. 10.1203/01.PDR.0000169976.20029.64.
Melville S, McNurlan MA, McHardy KC, Broom J, Milne E, Calder AG, Garlick PJ: The role of degradation in the acute control of protein balance in adult man: failure of feeding to stimulate protein synthesis as assessed by L-[1-C]leucine infusion. Metabolism: Clinical and Experimental. 1989, 38 (3): 248-255. 10.1016/0026-0495(89)90083-8.
Braziuniene I, Garlick J, Mileva I, Desikan V, Wilson TA, McNurlan M: Effect of insulin with oral nutrients on whole-body protein metabolism in growing pubertal children with type 1 diabetes. Pediatric Research. 2009, 65 (1): 109-112. 10.1203/PDR.0b013e3181894911.
Toth MJ, MacCoss MJ, Poehlman ET, Matthews DE: Recovery of
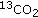 from infused [1-
from infused [1-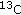 ]leucine and [1,2-
]leucine and [1,2- ]leucine in healthy humans. The American Journal of Physiology. 2001, 281 (2): E233-E241.
]leucine in healthy humans. The American Journal of Physiology. 2001, 281 (2): E233-E241.Mattews DE, Schwartz HP, Yang RD: Relationship of plasma leucine and α-ketoisocaproate during a L-[1-
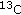 ]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism: Clinical and Experimental. 1982, 31 (11): 1105-1112. 10.1016/0026-0495(82)90160-3.
]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism: Clinical and Experimental. 1982, 31 (11): 1105-1112. 10.1016/0026-0495(82)90160-3.Golden MHN, Waterlow JC: Total protein synthesis in elderly people: a comparison of results with [
 ]glycine and [
]glycine and [ ]leucine. Clinical Science and Molecular Medicine. 1977, 53 (3): 277-288.
]leucine. Clinical Science and Molecular Medicine. 1977, 53 (3): 277-288.Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS: Oral leucine administration stimulates protein synthesis in rat skeletal muscle. Journal of Nutrition. 2005, 135 (3): 376-382.
Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA: Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. The American Journal of Physiology. 2005, 288 (5): E914-E921.
Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA: Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. The American Journal of Physiology. 2008, 295 (4): E868-E875.
Inchiostro S, Biolo G, Bruttomesso D, Fongher C, Sabadin L, Carlini M, Duner E, Tiengo A, Tessari P: Effects of insulin and amino acid infusion on leucine and phenylalanine kinetics in type 1 diabetes. The American Journal of Physiology. 1992, 262 (2): E203-E210.
Acknowledgments
The authors would like to thank the subjects and their families for participating in this research. They also thank the nursing staff of the General Clinical Research Center and Ms. Dawn Sasvary from the Core Lab for all their help in the completion of this work. This work was funded by grants from the National Institute of Health M01RR01710 to Stony Brook University Medical Center and M01RR00109 to the University of Vermont.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Desikan, V., Mileva, I., Garlick, J. et al. The Effect of Oral Leucine on Protein Metabolism in Adolescents with Type 1 Diabetes Mellitus. Int J Pediatr Endocrinol 2010, 493258 (2010). https://doi.org/10.1155/2010/493258
Received:
Accepted:
Published:
DOI: https://doi.org/10.1155/2010/493258






 -ketoisocaproate.
-ketoisocaproate.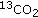 from infused [1-
from infused [1-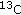 ]leucine and [1,2-
]leucine and [1,2- ]leucine in healthy humans. The American Journal of Physiology. 2001, 281 (2): E233-E241.
]leucine in healthy humans. The American Journal of Physiology. 2001, 281 (2): E233-E241.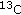 ]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism: Clinical and Experimental. 1982, 31 (11): 1105-1112. 10.1016/0026-0495(82)90160-3.
]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism: Clinical and Experimental. 1982, 31 (11): 1105-1112. 10.1016/0026-0495(82)90160-3. ]glycine and [
]glycine and [ ]leucine. Clinical Science and Molecular Medicine. 1977, 53 (3): 277-288.
]leucine. Clinical Science and Molecular Medicine. 1977, 53 (3): 277-288.