Abstract
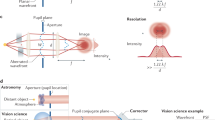
This contribution is another opportunity to acknowledge the influence of Roger Maynard on our research work when he pushed one of us (ACB) to explore the field of waves propagating in complex media rather than limiting ourselves to the wavelength scale of thermal waves or near field phenomena. Optical tomography is used for imaging in-depth scattering media such as biological tissues. Optical coherence tomography (OCT) plays an important role in imaging biological samples. Coupling OCT with adaptive optics (AO) in order to correct eye aberrations has led to cellular imaging of the retina. By using our approach called Full-Field OCT (FFOCT) we show that, with spatially incoherent illumination, the width of the point-spread function (PSF) that governs the resolution is not affected by aberrations that induce only a reduction of the signal level. We will describe our approach by starting with the PSF experimental data followed by a simple theoretical analysis, and numerical calculations. Finally full images obtained through or inside scattering and aberrating media will be shown.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
T. Wilson, in Confocal Microscopy (Academic Press: London, 1990), Vol. 426, pp. 1–64
W. Denk, J.H. Strickler, W.W. Webb, Two-photon laser scanning fluorescence microscopy, Science 248, 73 (1990)
D. Huang, E.A. Swanson, C.P. Lin, J.S. Schuman, W.G. Stinson, W. Chang, M.R. Hee, T. Flotte, K. Gregory, C.A. Puliafito, J.G. Fujimoto, Optical coherence tomography, Science 254, 1178 (1991)
N. Nassif, B. Cense, B.H. Park, S.H. Yun, T.C. Chen, B.E. Bouma, G.J. Tearney, J.F. de Boer, In vivo human retinal imaging by ultrahigh-speed spectral domain optical coherence tomography, Opt. Lett. 29, 480 (2004)
R. Huber, M. Wojtkowski, J.G. Fujimoto, Fourier Domain Mode Locking (FDML): A new laser operating regime and applications for optical coherence tomography, Opt. Express 14, 3225 (2006)
B.M. Hoeling, A.D. Fernandez, R.C. Haskell, E. Huang, W.R. Myers, D.C. Petersen, S.E. Ungersma, R.Wang, M.E. Williams, S.E. Fraser, An optical coherence microscope for 3-dimensional imaging in developmental biology, Opt. Express 6, 136 (2000)
Y. Zhang, J. Rha, R.S. Jonnal, D.T. Miller, Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina, Opt. Express 13, 4792 (2005)
S. Bourquin, P. Seitz, R.P. Salathé, Optical coherence topography based on a two-dimensional smart detector array, Opt. Lett. 26, 512 (2001)
E. Bordenave, E. Abraham, G. Jonusauskas, N. Tsurumachi, J. Oberlé, C. Rullière, P.E. Minot, M. Lassègues, J.E. Surlève Bazeille, Wide-field optical coherence tomography: imaging of biological tissues, Appl. Opt. 41, 2059 (2002)
M. Laubscher, M. Ducros, B. Karamata, T. Lasser, R. Salathé, Video-rate three-dimensional optical coherence tomography, Opt. Express 10, 429 (2002)
B. Karamata, P. Lambelet, M. Laubscher, R.P. Salathé, T. Lasser, Spatially incoherent illumination as a mechanism for cross-talk suppression in wide-field optical coherence tomography, Opt. Lett. 29, 736 (2004)
L. Vabre, A. Dubois, A.C. Boccara, Thermal-light full-field optical coherence tomography, Opt. Lett., 27, 530 (2002)
LLTech SAS, France, http://www.lltechimaging.com/
P. Xiao, M. Fink, A.C. Boccara, Full-field spatially incoherent illumination interferometry: a spatial resolution almost insensitive to aberrations, Opt. Lett. 41, 3920 (2016)
M. Born, E. Wolf, Principles of optics: electromagnetic theory of propagation, interference and diffraction of light (CUP Archive, 2000)
J.W. Goodman, Introduction to Fourier optics (Roberts and Company Publishers, 2005)
C.W. McCutchen, Generalized Source and the van Cittert–Zernike Theorem: A Study of the Spatial Coherence Required for Interferometry, J. Opt. Soc. Am. 56, 727 (1966)
H. Babcock, The possibility of compensating atmospheric seeing, Pub. Astr. Soc. Pac. 65, 229 (2010)
G. Rousset, J.C. Fontanella, P. Kern, F. Rigaut, First diffraction-limited astronomical images with adaptive optics, Astron. Astrophys. 230, L29 (1990)
J.A. Kubby, Adaptive Optics for Biological Imaging (CRC Press, 2013)
P. Godara, A.M. Dubis, A. Roorda, J.L. Duncan, J. Carroll, Adaptive optics retinal imaging: emerging clinical applications, Optom. Vis. Sci. 87, 930 (2010)
J. Porter, H.M. Queener, J.E. Lin, K. Thorn, A. Awwal (ed.), Adaptive Optics for Vision Science (John Wiley & Sons, Inc., 2006)
D.R. Williams, Imaging single cells in the living retina, Vision Res. 51, 1379 (2011)
J. Liang, D.R. Williams, D.T. Miller, Supernormal vision and high-resolution retinal imaging through adaptive optics, J. Opt. Soc. Am. A 14, 2884 (1997)
A. Roorda, F. Romero-Borja, W. Donnelly III, H. Queener, T. Hebert, M. Campbell, Adaptive optics scanning laser ophthalmoscopy, Opt. Express 10, 405 (2002)
Y. Zhang, A Roorda, Evaluating the lateral resolution of the adaptive optics scanning laser ophthalmoscope, J. Biomed. Opt. 11, 14002 (2006)
D. Merino, J.L. Duncan, P. Tirubeedhula, A. Roorda, Observation of cone and rod photoreceptors in normal subjects and patients using a new generation adaptive optics scanning laser ophthalmoscope, Biomed. Opt. Express 2, 2189 (2011)
Y. Zhang, B. Cense, J. Rha, R.S. Jonnal, W. Gao, R.J. Zawadzki, J.S. Werner, S. Jone, S. Olivier, D.T. Miller, High-speed volumetric imaging of cone photoreceptoes with adaptive optics spectral-domain optical coherence tomography, Opt. Express 14, 4380 (2006)
R.J. Zawadzki, S.M. Jones, S.S. Olivier, M. Zhao, B.A. Bower, J.A. Izatt, S. Choi, S. Laut, J.S. Werner, Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging, Opt. Express 13, 8532 (2005)
O.P. Kocaoglu, S. Lee, R.S. Jonnal, Q. Wang, A.E. Herde, J.C. Derby, W. Gao, D.T. Miller, Imaging cone photoreceptors in three dimensions and in time using ultrahigh resolution optical coherence tomography with adaptive optics, Biomed. Opt. Express 2, 748 (2011)
Y. Zhang, J. Rha, R. Jonnal, D. Miller, Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina, Opt. Express 13, 4792 (2005)
E.J. Fernández, B. Hermann, B. Povazay, A. Unterhuber, H. Sattmann, B. Hofer, P.K. Ahnelt, W. Drexler, Ultrahigh resolution optical coherence tomography and pancorrection for cellular imaging of the living human retina, Opt. Express 16, 11083 (2008)
E.J. Fernández, B. Povazay, B. Hermann, A. Unterhuber, H. Sattmann, P.M. Prieto, R. Leitgeb, P. Ahnelt, P. Artal, W. Drexler, Three-dimensional AO ultrahigh-resolution optical coherence tomography using a liquid crystal spatial light modulator, Vision Res. 45, 3432 (2005)
O.P. Kocaoglu, R.D. Ferguson, R.S. Jonnal, Z. Liu, Q. Wang, D.X. Hammer, D.T. Miller, Adaptive optics optical coherence tomography with dynamic retinal tracking, Biomed. Opt. Express 5, 2262 (2014)
K.S.K. Wong, Y. Jian, M. Cua, S. Bonora, R.J. Zawadzki, M.V. Sarunic, In vivo imaging of human photoreceptor mosaic with wavefront sensorless adaptive optics optical coherence tomography, Biomed. Opt. Express 6, 580 (2015)
M. Rueckel, J.A. Mack-Bucher, W. Denk, Adaptive wavefront correction in two-photon microscopy using coherence-gated wavefront sensing, PNAS 103, 17137 (2006)
S.H. Lee, J.S. Werner, R.J. Zawadzki, Improved visualization of outer retinal morphology with aberration cancelling reflective optical design for adaptive optics – optical coherence tomography, Biomed. Opt. Express 4, 2508 (2013)
J. Porter, A. Guirao, I.G. Cox, D.R. Williams, Monochromatic aberrations of the human eye in a large population, JOSA A 18, 1793 (2001)
J.F. Castejón-Mochón, N. López-Gil, A. Benito, P. Artal, Ocular wave-front aberration statistics in a normal young population, Vision Res. 42, 1611 (2002)
X. Hong, L. Thibos, A. Bradley, D. Miller, X. Cheng, N. Himebaugh, Statistics of aberrations among healthy young eyes, in Vision Science and its Applications, edited by A. Sawchuk, Vol. 53 of OSA Trends in Optics and Photonics (Optical Society of America, 2001), p. SuA5
D3128 Spatial Light Modulator, Meadowlark Optics, http://www.meadowlark.com/
G.D. Love, Wave-front correction and production of Zernike modes with a liquid-crystal spatial light modulator, Appl. Opt. 36, 1517 (1997)
A. Vyas, M.B. Roopashree, R.K. Banyal, B.R. Prasad, Spatial Light Modulator for wavefront correction, arXiv:0909.3413 (2009)
L.N. Thibos, A. Bradley, Use of liquid-crystal adaptive-optics to alter the refractive state of the eye, Optometry Vision Sci. 74, 581 (1997)
F. Vargas-Martin, P.M. Prieto, P. Artal, Correction of the aberrations in the human eye with a liquid-crystal spatial light modulator: limits to performance, JOSA A 15, 2552 (1998)
D.T. Miller, L.N. Thibos, X. Hong, Requirements for segmented correctors for diffraction-limited performance in the human eye, Opt. Express 13, 275 (2005)
P.N. Marsh, D. Burns, J.M. Girkin, Practical implementation of adaptive optics in multiphoton microscopy, Opt. Express 11, 1123 (2003)
L. Sherman, J.Y. Ye, O. Albert, T.B. Norris, Adaptive correction of depth-induced aberrations in multiphoton scanning microscopy using a deformable mirror, J. Microsc-Oxford 206, 65 (2002)
S. Zommer, E.N. Ribak, S.G. Lipson, J. Adler, Simulated annealing in ocular adaptive optics, Opt. Lett. 31, 939 (2006)
N. Ji, D.E. Milkie, E. Betzig, Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues, Nat. Meth. 7, 141 (2009)
A. Dubois, G. Moneron, A.C. Boccara, Thermal-light full-field optical coherence tomography in the 1.2μm wavelength region. Opt. Commun. 266, 738 (2006)
S. Labiau, G. David, S. Gigan, A.C. Boccara, Defocus test and defocus correction in full-field optical coherence tomography, Opt. Lett. 34, 1576 (2009)
J. Binding, J.B. Arous, J. Leger, S. Gigan, C. Boccara, L. Bourdieu, Brian refractive index measured in vivo with high-NA defocus-corrected full-field OCT and consequences for two-photon microscopy, Opt. Express 19, 4833 (2011)
J. Wang, J. Leger, J. Binding, A.C. Boccara, S. Gigan, L. Bourdieu, Measuring aberrations on the rat brain by coherence-gated wavefront sensing using a Linnik interferometer, Biomed. Opt. Expess 3, 2510 (2012)
P. Xiao, M. Fink, A.C. Boccara, Adapive optics full-field optical coherence tomography, submitted to Journal of Biomedical Optics
R.J. Noll, Zernike polynomials and atmospheric turbulence, JOsA 66, 207 (1976)
G. Dai, Wavefront Optics for Vision Correction (SPIE Press, 2008)
S. Bonora, R. J. Zawadzki, Wavefront sensorless modal deformable mirror correction in adaptive optics: optical coherence tomography, Opt. Lett. 38, 4801 (2013)
D. Debarre, M.J. Booth, T. Wilson, Image based adaptive optics through optimization of low spatial frequencies, Opt. Express 15, 8176 (2007)
J. Mertz, H. Paudel, T.G. Bifano, Field of view advantage of conjugate adaptive optics in microscopy applications, Appl. Opt. 54, 3498 (2015)
C.K. Hitzenberger, A. Baumgartner, W. Drexler, A.F. Fercher, Dispersion effects in partial coherence interferometry: implications for intraocular ranging, J. Biomed. Opt. 4, 144 (1999)
ROWE Technical Design, Inc., http://www.rowetechnical.com/tpme.html
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xiao, P., Fink, M., Gandjbakhche, A.H. et al. A resolution insensitive to geometrical aberrations by using incoherent illumination and interference imaging. Eur. Phys. J. Spec. Top. 226, 1603–1621 (2017). https://doi.org/10.1140/epjst/e2017-70001-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjst/e2017-70001-7