Abstract
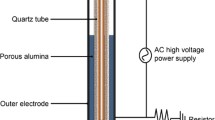
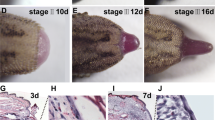
Atmospheric pressure plasma is a partially ionized gas composed of neutral and charged particles, including electrons and ions, as well as reactive oxygen species (ROS). Recently, it is utilized as possible therapy in oncology, sterilization, skin diseases, wound healing and tissue regeneration. In this study we focused on effect of plasma exposure on tail regeneration of tadpoles, Xenopus leavis with special emphasis on role of ROS, antioxidant defenses and morphological features of the regenerate. When amputated region of the tail was exposed to the helium plasma it resulted in a faster rate of growth, elevated ROS and increase in antioxidant enzymes in the regenerate compared to that of untreated control. An increase in nitric oxide (free radical) as well as activity of nitric oxide synthase(s) were observed once the cells of the regeneration blastema – a mass of proliferating cells are ready for differentiation. Microscopically the cells of the regenerate of plasma treated tadpoles show altered morphology and characteristics of cellular hypoxia and oxidative stress. We summarize that plasma exposure accelerates the dynamics of wound healing and tail regeneration through its effects on cell proliferation and differentiation as well as angiogenesis mediated through ROS signaling.
Similar content being viewed by others
References
D. Dobrynin, G. Fridman, G. Friedman, A. Fridman, New J. Phys. 11, 115020 (2009)
G. Fridman, A.D. Brooks, M. Balasubramanian, A. Fridman, A. Gutsol, V.N. Vasilets, H. Ayan, G. Friedman, Plasma Proc. Poly. 4, 370 (2007)
D. Kim, B. Gweon, D. Kim, W. Choe, J. Shin, in 13th International Conference on Biomedical Engineering (Springer, 2009), p. 355
R. Ma, H. Feng, Y. Liang, Q. Zhang, Y. Tian, B. Su, J. Zhang, J. Fang, J. Phys. D 46, 285401 (2013)
G. Fridman, G. Friedman, A. Gutsol, A.B. Shekhter, V.N. Vasilets, A. Fridman, Plasma Proc. Polym. 5, 503 (2008)
A. Lupu, N. Georgescu, Romanian Rep. Phys. 65, 219 (2013)
M. Mittal, M.R. Siddiqui, K. Tran, S.P. Reddy, A.B. Malik, Antioxidants & Redox Signaling 20, 1126 (2014)
E. Barbieri, P. Sestili, J. Signal. Transduct 2012, 982794 (2012)
T.S. Gechev, F. Van Breusegem, J.M. Stone, I. Denev, C. Laloi, Bioessays 28, 1091 (2006)
F.J. Gonzalez, Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 569, 101 (2005)
R. Harrison, Drug Metab. Rev. 36, 363 (2004)
C.F. Mueller, K. Laude, J.S. McNally, D.G. Harrison, Arterioscler. Thromb. Vasc. Biol. 25, 274 (2005)
K.A. Pritchard Jr, A.W. Ackerman, E.R. Gross, D.W. Stepp, Y. Shi, J.T. Fontana, J.E. Baker, W.C. Sessa, J. Biol. Chem. 276, 17621 (2001)
V.J. Thannickal, B.L. Fanburg, Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L1005 (2000)
A.S. Tseng, M. Levin, J. Dent. Res. 87, 806 (2008)
M. Mochii, Y. Taniguchi, I. Shikata, Dev. Growth Differ. 49, 155 (2007)
J. Slack, G. Lin, Y. Chen, Cellular Mol. Life Sci. 65, 54 (2008)
N.R. Love, Y. Chen, S. Ishibashi, P. Kritsiligkou, R. Lea, Y. Koh, J.L. Gallop, K. Dorey, E. Amaya, Nat. Cell Biol. 15, 222 (2013)
A. Franchini, E. Bertolotti, Cell Tissue Res. 344, 261 (2011)
M. Ziche, L. Morbidelli, J. Neurooncol. 50, 139 (2000)
P.D. Nieuwkoop, J. Faber, Normal Table of Xenopus Laevis (Daudin) : A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis (North-Holland, Amsterdam, 1967)
N. Masoud, K. Martus, M. Figus, K. Becker, Contrib. Plasma Phys. 45, 32 (2005)
A. Ibabe, M. Grabenbauer, E. Baumgart, A. Völkl, H.D. Fahimi, M.P. Cajaraville, Acta Histochem. 106, 11 (2004)
G.P. Bienert, J.K. Schjoerring, T.P. Jahn, Biochimica Et Biophysica Acta (BBA) - Biomembranes 1758, 994 (2006)
N. Suzuki, R. Mittler, Free Radical Biol. Medicine 53, 2269 (2012)
J.M. Li, A.M. Shah, J. Biol. Chem. 277, 19952 (2002)
N. Peunova, V. Scheinker, H. Cline, G. Enikolopov, J. Neurosci. 21, 8809 (2001)
R. Saini, S. Patel, R. Saluja, A.A. Sahasrabuddhe, M.P. Singh, S. Habib, V.K. Bajpai, M. Dikshit, J. Leukoc. Biol. 79, 519 (2006)
M.G. Kong, G. Kroesen, G. Morfill, T. Nosenko, T. Shimizu, J. Van Dijk, J. Zimmermann, New J. Physics 11, 115012 (2009)
N. Ale-Agha, L. Klotz, Signal Transduction 1, S45 (2007)
B.L. Upham, K.S. Kang, H.Y. Cho, J.E. Trosko, Carcinogenesis 18, 37 (1997)
V. Kumar, A.K. Abbas, J.C. Aster, Robbins Basic Pathology (Elsevier Health Sciences, 2012)
S.A. Eming, M. Hammerschmidt, T. Krieg, A. Roers, in Seminars in cell & developmental biology (Elsevier, 2009), p. 517
J. Kanta, Acta Medica (Hradec Kralove) 54, 97 (2011)
R.C. Melo, H. D’Avila, H.C. Wan, P.T. Bozza, A.M. Dvorak, P.F. Weller, J. Histochem. Cytochem. 59, 540 (2011)
S. Arndt, P. Unger, E. Wacker, T. Shimizu, J. Heinlin, Y. Li, H.M. Thomas, G.E. Morfill, J.L. Zimmermann, A. Bosserhoff, PloS One 8, e79325 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivie, A., Martus, K. & Menon, J. Atmospheric pressure plasma accelerates tail regeneration in tadpoles Xenopus laevis . Eur. Phys. J. Spec. Top. 226, 2859–2871 (2017). https://doi.org/10.1140/epjst/e2016-60243-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjst/e2016-60243-3