Abstract
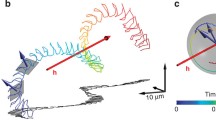
Many cells and microorganisms have evolved a motility apparatus to explore their surroundings. For guidance, these biological microswimmers rely on physical and chemical cues that are transduced by cellular pathways into directed movement – a process called taxis. Only few biological microswimmers have been studied as detailed as sperm from sea urchins. Sperm and eggs are released into the seawater. To enhance the chances of fertilization, eggs release chemical factors – called chemoattractants – that establish a chemical gradient and, thereby, guide sperm to the egg. Sea urchin sperm constitute a unique model system for understanding cell navigation at every level: from molecules to cell behaviours. We will outline the chemotactic signalling pathway of sperm from the sea urchin Arbacia punctulata and discuss how signalling controls navigation in a chemical gradient. Finally, we discuss recent insights into sperm chemotaxis in three dimensions (3D).
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
E.M. Purcell, Am. J. Phys. 45, 3 (1977)
L. Alvarez, B.M. Friedrich, G. Gompper, U.B. Kaupp, Trends Cell Biol. 24, 198 (2014)
R.G. Winkler, Eur. Phys. J. Special Topics 225, 2079 (2016)
M. Pichlo, S. Bungert-Plumke, I. Weyand, R. Seifert, W. Bönigk, T. Strünker, N.D. Kashikar, N. Goodwin, A. Muller, H.G. Körschen, et al., J. Cell Biol. 206, 541 (2014)
R. Seifert, M. Flick, W. Bonigk, L. Alvarez, C. Trotschel, A. Poetsch, A. Muller, N. Goodwin, P. Pelzer, N.D. Kashikar, et al., EMBO J. 34, 379 (2015)
T. Strünker, L. Alvarez, U.B. Kaupp, Curr. Opin. Neurobiol. 34, 110 (2015)
L. Alvarez, L. Dai, B.M. Friedrich, N.D. Kashikar, I. Gregor, R. Pascal, U.B. Kaupp, J. Cell Biol. 196, 653 (2012)
J.F. Jikeli, L. Alvarez, B.M. Friedrich, L.G. Wilson, R. Pascal, R. Colin, M. Pichlo, A. Rennhack, C. Brenker, U.B. Kaupp, Nat. Commun. 6, 7985 (2015)
K. Shiba, S.A. Baba, T. Inoue, M. Yoshida, Proc. Natl. Acad. Sci. U.S.A. 105, 19312 (2008)
U.B. Kaupp, J. Gen. Physiol. 140, 583 (2012)
D. Carre, C. Sardet, Biol. Cell 40, 128 (1981)
G.E. Ward, C.J. Brokaw, D.L. Garbers, V.D. Vacquier, J. Cell Biol. 101, 2324 (1985)
A. Guerrero, T. Nishigaki, J. Carneiro, T. Yoshiro, C.D. Wood, A. Darszon, Dev. Biol. 344, 52 (2010)
N.D. Kashikar, L. Alvarez, R. Seifert, I. Gregor, O. Jackle, M. Beyermann, E. Krause, U.B. Kaupp, J. Cell Biol. 198, 1075 (2012)
U.B. Kaupp, J. Solzin, E. Hildebrand, J.E. Brown, A. Helbig, V. Hagen, M. Beyermann, F. Pampaloni, I. Weyand, Nat. Cell Biol. 5, 109 (2003)
D.L. Garbers, D.G. Lowe, L.J. Dangott, M. Chinkers, D.S. Thorpe, J.K. Bentley, C.S. Ramarao, D.V. Goeddel, S. Singh, Cold Spring Harbor Symp. Quant. Biol. LIII, 993 (1988)
W. Bönigk, A. Loogen, R. Seifert, N.D. Kashikar, C. Klemm, E. Krause, V. Hagen, E. Kremmer, T. Strünker, U.B. Kaupp, Sci. Signal. 2, ra68 (2009)
T. Strünker, I. Weyand, W. Bönigk, Q. Van, A. Loogen, J.E. Brown, N.D. Kashikar, V. Hagen, E. Krause, U.B. Kaupp, Nat. Cell Biol. 8, 1149 (2006)
B.E. Galindo, J.L. de la Vega-Beltran, P. Labarca, V.D. Vacquier, A. Darszon, Biochem. Biophys. Res. Commun. 354, 668 (2007)
H.C. Lee, D.L. Garbers, J. Biol. Chem. 261, 16026 (1986)
M. Nomura, V.D. Vacquier, Cell Motil. Cytoskel. 63, 582 (2006)
D. Wang, S.M. King, T.A. Quill, L.K. Doolittle, D.L. Garbers, Nat. Cell Biol. 5, 1117 (2003)
R. Gauss, R. Seifert, U.B. Kaupp, Nature 393, 583 (1998)
H.J. Gunaratne, V.D. Vacquier, FEBS Lett. 580, 3900 (2006)
Y.H. Su, V.D. Vacquier, Mol. Biol. Cell 17, 114 (2006)
Y.-H. Su, V.D. Vacquier, Proc. Natl. Acad. Sci. USA 99, 6743 (2002)
J.R. Maddock, L. Shapiro, Science 259, 1717 (1993)
D. Bray, M.D. Levin, C.J. Morton-Firth, Nature 393, 85 (1998)
T.A.J. Duke, D. Bray, PNAS 96, 10104 (1999)
J.E. Gestwicki, L.L. Kiessling, Nature 415, 81 (2002)
V. Sourjik, H.C. Berg, Nature 428, 437 (2004)
E.N.J. Pugh, T.D. Lamb In Handbook of Biological Physics, Volume 3, edited by D.G. Stavenga, W.J. DeGrip and E.N.J. Pugh, (North-Holland, Elsevier Science B.V., 2000), p. 183
K.W. Yau, R.C. Hardie, Cell 139, 246 (2009)
U.B. Kaupp, Nat. Rev. Neurosci. 11, 188 (2010)
A. Huber, P. Sander, A. Gobert, M. Bahner, R. Hermann, R. Paulsen, EMBO J. 15, 7036 (1996)
S. Tsunoda, J. Sierralta, Y. Sun, R. Bodner, E. Suzuki, A. Becker, M. Socolich, C.S. Zuker, Nature 388, 243 (1997)
K. Scott, C.S. Zuker, Nature 395, 805 (1998)
T.C. Rich, K.A. Fagan, H. Nakata, J. Schaack, D.M.F. Cooper, J.W. Karpen, J. Gen. Physiol. 116, 147 (2000)
T.C. Rich, T.E. Tse, J.G. Rohan, J. Schaack, J.W. Karpen, J. Gen. Physiol. 118, 63 (2001)
M. Gunkel, J. Schöneberg, W. Alkhaldi, S. Irsen, F. Noe, U.B. Kaupp, A. Al-Amoudi, Structure 23, 628 (2015)
D. Fotiadis, Y. Liang, S. Filipek, D.A. Saperstein, A. Engel, K. Palczewski, Nature 421, 127 (2003)
H.C. Berg, E.M. Purcell, Biophys. J. 20, 193 (1977)
H.C. Berg, Random Walks in Biology (Princeton University Press, Princeton, New Jersey, 1993)
M. Li, G.L. Hazelbauer, J. Bacteriol. 186, 3687 (2004)
V.J. Cannistraro, G.D. Glekas, C.V. Rao, G.W. Ordal, J. Bacteriol. 193, 3220 (2011)
V. Sourjik, N.S. Wingreen, Curr. Opin. Cell Biol. 24, 262 (2012)
G.E. Ward, G.W. Moy, V.D. Vacquier, Adv. Exp. Med. Biol. 207, 359 (1986)
N. Suzuki, H. Shimomura, E.W. Radany, C.S. Ramarao, G.E. Ward, J.K. Bentley, D.L. Garbers, J. Biol. Chem. 259, 14874 (1984)
T. Doan, A. Mendez, P.B. Detwiler, J. Chen, F. Rieke, Science 313, 530 (2006)
A. Mendez, M.E. Burns, A. Roca, J. Lem, L.-W. Wu, M.I. Simon, D.A. Baylor, J. Chen, Neuron 28, 153 (2000)
G.G. Whitlock, T.D. Lamb, Neuron 23, 337 (1999)
O.P. Gross Jr.E.N. Pugh, M.E. Burns, Biophys. J. 102, 1775 (2012)
O.P. Gross Jr., E.N. Pugh, M.E. Burns, Neuron 76, 370 (2012)
G. Caruso, P. Bisegna, D. Andreucci, L. Lenoci, V.V. Gurevich, H.E. Hamm, E. DiBenedetto, Proc. Natl. Acad. Sci. USA 108, 7804 (2011)
V. Arshavsky, M. Burns, Cellular Logistics, e28680 (2014)
N.J. Cook, L.L. Molday, D. Reid, U.B. Kaupp, R.S. Molday, J. Biol. Chem. 264, 6996 (1989)
K.W. Yau, D.A. Baylor, Annu. Rev. Neurosci. 12, 289 (1989)
U.B. Kaupp, T. Niidome, T. Tanabe, S. Terada, W. Bönigk, W. Stühmer, N.J. Cook, K. Kangawa, H. Matsuo, T. Hirose, et al., Nature 342, 762 (1989)
C. Biskup, J. Kusch, E. Schulz, V. Nache, F. Schwede, F. Lehmann, V. Hagen, K. Benndorf, Nature 446, 440 (2007)
P.V. Lishko, Y. Kirichok, D. Ren, B. Navarro, J.J. Chung, D.E. Clapham, Annu. Rev. Physiol. 74, 453 (2012)
P.V. Lishko, I.L. Botchkina, Y. Kirichok, Nature 471, 387 (2011)
T. Strünker, N. Goodwin, C. Brenker, N.D. Kashikar, I. Weyand, R. Seifert, U.B. Kaupp, Nature 471, 382 (2011)
M.R. Miller, N. Mannowetz, A.T. Iavarone, R. Safavi, E.O. Gracheva, J.F. Smith, R.Z. Hill, D.M. Bautista, Y. Kirichok, P.V. Lishko, Science 352, 555 (2016)
U.B. Kaupp, R. Seifert, Annu. Rev. Physiol. 63, 235 (2001)
M. Biel, C. Wahl-Schott, S. Michalakis, X. Zong, Physiological Rev. 89, 847 (2009)
J.J.B. Jack, D. Noble, R.W. Tsien, Electric Current Flow in Excitable Cells, (Oxford University Press, 1983)
B. Navarro, Y. Kirichok, D.E. Clapham, Proc. Natl. Acad. Sci. U.S.A. 104, 7688 (2007)
V.D. Vacquier, A. Loza-Huerta, J. Garcia-Rincon, A. Darszon, C. Beltran, Biochim. Biophys. Acta. 1842, 2621 (2014)
T. Nishigaki, O. Jose, A.L. Gonzalez-Cota, F. Romero, C.L. Trevino, A. Darszon, Biochem. Biophys. Res. Commun. 450, 1149 (2014)
A. Darszon, T. Nishigaki, C. Beltran, C.L. Trevino, Physiological Rev. 91, 1305 (2011)
T. Nishigaki, K. Chiba, W. Miki, M. Hoshi, Zygote 4, 237 (1996)
M. Matsumoto, J. Solzin, A. Helbig, V. Hagen, S. Ueno, O. Kawase, Y. Maruyama, M. Ogiso, M. Godde, H. Minakata, et al., Dev. Biol. 260, 314 (2003)
M. Böhmer, Q. Van, I. Weyand, V. Hagen, M. Beyermann, M. Matsumoto, M. Hoshi, E. Hildebrand, U.B. Kaupp, EMBO J. 24, 2741 (2005)
S. Fechner, L. Alvarez, W. Bönigk, A. Müller, T.K. Berger, R. Pascal, C. Trötschel, A. Poetsch, G. Stölting, K.R. Siegfried, et al., eLife 4, e07624 (2015)
X. Cai, D.E. Clapham, PloS one 3, e3569 (2008)
V. Hagen, S. Frings, J. Bendig, D. Lorenz, B. Wiesner, U.B. Kaupp, Angew. Chem. Int. Ed. 41, 3625 (2002)
V. Hagen, J. Bendig, S. Frings, T. Eckardt, S. Helm, D. Reuter, U.B. Kaupp, Angew. Chem. Int. Ed. 40, 1046 (2001)
J. Elgeti, U.B. Kaupp, G. Gompper, Biophys. J. 99, 1018 (2010)
J. Elgeti, G. Gompper, Eur. Phys. J. Special Topics 225, 3033 (2016)
D.M. Woolley, Reprod. 126, 259 (2003)
B.M. Friedrich, F. Julicher, Proc. Natl. Acad. Sci. U.S.A. 104, 13256 (2007)
G.S. Fraenkel, D.L. Gunn, The Orientation of Animals: Kinesis, Taxes and Compass Reactions (Dover Publications, Inc., New York, 1961)
R. Thar, T. Fenchel, Appl. Environ. Microbiol. 67, 3299 (2001)
J. Riedl, M. Louis, Curr. Biol. 22, R867 (2012)
S. Faumont, T.H. Lindsay, S.R. Lockery, Curr. Opin. Neurobiol. 22, 580 (2012)
S.R. Lockery, Curr. Opin. Neurobiol. 21, 782 (2011)
J. Bussmann, E. Raz, EMBO J. 34, 1309 (2015)
H.C. Crenshaw, Helical Orientation – a Novel Mechanism for the Orientation of Microorganisms. In Lecture Notes in Biomathematics, edited by W. Alt and G. Hofmann (Berlin, Heidelberg, New York, Springer, 1990), p. 361
C.J. Brokaw, J. Cell Biol. 82, 401 (1979)
C.B. Lindemann, K.A. Lesich, Methods Mol. Biol. 586, 337 (2009)
C.B. Lindemann, T.K. Gardner, E. Westbrook, K.S. Kanous, Cell Motil. Cytoskel. 20, 316 (1991)
C.B. Lindemann, J.S. Goltz, Cell Motil. Cytoskel. 10, 420 (1988)
C.D. Wood, T. Nishigaki, T. Furuta, S.A. Baba, A. Darszon, J. Cell Biol. 169, 725 (2005)
Y. Kambara, K. Shiba, M. Yoshida, C. Sato, K. Kitajima, C. Shingyoji, Cell Struct. Funct. 36, 69 (2011)
V. Torre, J.F. Ashmore, T.D. Lamb, A. Menini, J. Neurosci. the official J. Soc. Neurosci. 15, 7757 (1995)
K. Yoshimura, R. Kamiya, Plant Cell Physiol. 42, 665 (2001)
M. Delling, P.G. DeCaen, J.F. Doerner, S. Febvay, D.E. Clapham, Nature 504, 311 (2013)
P.G. DeCaen, M. Delling, T.N. Vien, D.E. Clapham, Nature 504, 315 (2013)
G.J. Pazour, N. Agrin, J. Leszyk, G.B. Witman, J. Cell Biol. 170, 103 (2005)
C.G. DiPetrillo, E.F. Smith, J. Cell Biol. 189, 601 (2010)
C. DiPetrillo, E. Smith, Methods Cell Biol. 92, 163 (2009)
S.M. King, Cytoskeleton (Hoboken) 67, 207 (2010)
H. Suzuki, T.R. Thiele, S. Faumont, M. Ezcurra, S.R. Lockery, W.R. Schafer, Nature 454, 114 (2008)
G. Corkidi, B. Taboada, C.D. Wood, A. Guerrero, A. Darszon, Biochem. Biophys. Res. Commun. 373, 125 (2008)
H.C. Crenshaw, Amer.Zool. 36, 608 (1996)
L. Marques, A. de Almeida, Auton. Robot. 20, 183 (2006)
J. Espinal, M. Aldana, A. Guerrero, C. Wood, A. Darszon, G. Martinez-Mekler, PloS one 6, e22619 (2011)
J. Espinal-Enriquez, A. Darszon, A. Guerrero, G. Martinez-Mekler, PloS one 9, e104451 (2014)
M.E. Burns, E.N.J. Pugh, Physiol. 25, 72 (2010)
G.C. Faas, S. Raghavachari, J.E. Lisman, I. Mody, Nat. Neurosci. 14, 301 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kaupp, U., Alvarez, L. Sperm as microswimmers – navigation and sensing at the physical limit. Eur. Phys. J. Spec. Top. 225, 2119–2139 (2016). https://doi.org/10.1140/epjst/e2016-60097-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjst/e2016-60097-1