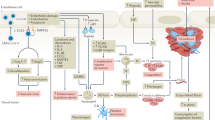
Abstract
Patients infected with COVID-19 may experience significant and long-lasting changes in the mechanical and rheological properties of their blood cells, leading to microvascular dysfunction and other vascular complications such as microthrombosis. In this study, we perform detailed computational simulations to investigate the fibrinogen-dependent changes in microvascular blood flow. First, we develop a coarse-grained molecular model of plasma fibrinogen to investigate the correlation between fibrinogen concentration and plasma viscosity. Our simulation results show that plasma viscosity increases exponentially with fibrinogen concentration. We then use a coarse-grained RBC model to quantify the fibrinogen-dependent aggregation strength of RBC doublets and compare it with available experimental results. Next, we probe the effect of fibrinogen concentration on COVID-19 blood viscosity. Our simulation results show that increased plasma viscosity and blood cell aggregation are responsible for elevated blood viscosity. Finally, we quantify the alterations in microvascular blood flow in response to changes in cell adhesion. We find that the recruitment of WBCs and platelets would slow blood flow. The WBC–platelet adhesive interaction exacerbates the blockages, forming a complete blood occlusion at relatively low blood velocities. As blood velocities increase, the larger clusters of blood cells occluded by cell adhesion are more likely to dislodge from the site of inflammation. This computational study advances our understanding of the complex cell–cell interactions that influence microvascular blood flow. It highlights the importance of fibrinogen-induced changes in plasma viscosity, blood cell aggregation and adhesion in the risk of microvascular complications in patients with COVID-19.







Similar content being viewed by others
Data availability
The corresponding author’s data supporting this study’s findings are available upon reasonable request. This manuscript has data included as electronic supplementary material.
References
A.S. Popel, P.C. Johnson, Microcirculation and hemorheology. Annu. Rev. Fluid Mech. 37, 43–69 (2005)
J. Sun, K. Han, M. Xu, L. Li, J. Qian, L. Li, X. Li, Blood viscosity in subjects with type 2 diabetes mellitus: roles of hyperglycemia and elevated plasma fibrinogen. Front. Physiol. 13, 827428 (2022)
G. Goshua, A.B. Pine, M.L. Meizlish, C.-H. Chang, H. Zhang, P. Bahel, A. Baluha, N. Bar, R.D. Bona, A.J. Burns et al., Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 7, 575–582 (2020)
R. Escher, N. Breakey, B. Lämmle, Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 190, 62 (2020)
V.S.K. Edul, J.F.C. Eguillor, G. Ferrara, E. Estenssoro, D.S.P. Siles, C.E. Cesio, A. Dubin, Microcirculation alterations in severe COVID-19 pneumonia. J. Crit. Care 61, 73–75 (2021)
H. Su, M. Yang, C. Wan, L.-X. Yi, F. Tang, H.-Y. Zhu, F. Yi, H.-C. Yang, A.B. Fogo, X. Nie et al., Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in china. Kidney Int. 98, 219–227 (2020)
M.A. Gertz, R.A. Kyle, Hyperviscosity syndrome. J. Intensive Care Med. 10, 128–141 (1995)
S. Chien, S. Usami, R.J. Dellenback, M.I. Gregersen, Blood viscosity: influence of erythrocyte deformation. Science 157, 827–829 (1967)
S. Chien, S. Usami, R.J. Dellenback, M.I. Gregersen, Shear-dependent deformation of erythrocytes in rheology of human blood. Am. J. Physiol. 219, 136–142 (1970)
S. Chien, Shear dependence of effective cell volume as a determinant of blood viscosity. Science 168, 977–979 (1970)
C.L. Maier, A.D. Truong, S.C. Auld, D.M. Polly, C.-L. Tanksley, A. Duncan, COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 395, 1758–1759 (2020)
A. Berzuini, C. Bianco, A.C. Migliorini, M. Maggioni, L. Valenti, D. Prati, Red blood cell morphology in patients with COVID-19-related anaemia. Blood Transfus. 19, 34 (2021)
E. Nader, C. Nougier, C. Boisson, S. Poutrel, J. Catella, F. Martin, J. Charvet, S. Girard, S. Havard-Guibert, M. Martin et al., Increased blood viscosity and red blood cell aggregation in patients with COVID-19. Am. J. Hematol. 97, 283–292 (2022)
K. Lee, M. Kinnunen, M.D. Khokhlova, E.V. Lyubin, A.V. Priezzhev, I. Meglinski, A.A. Fedyanin, Optical tweezers study of red blood cell aggregation and disaggregation in plasma and protein solutions. J. Biomed. Opt. 21, 035001 (2016)
R.L. Clark, Genesis of placental sequestration in malaria and possible targets for drugs for placental malaria. Birth Defects Res. 111, 569–583 (2019)
L.R. Languino, J. Plescia, A. Duperray, A.A. Brian, E.F. Plow, J.E. Geltosky, D.C. Altieri, Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell 73, 1423–1434 (1993)
L. Nicolai, A. Leunig, S. Brambs, R. Kaiser, T. Weinberger, M. Weigand, M. Muenchhoff, J.C. Hellmuth, S. Ledderose, H. Schulz et al., Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 142, 1176–1189 (2020)
A. Le Joncour, L. Biard, M. Vautier, H. Bugaut, A. Mekinian, G. Maalouf, M. Vieira, A.-G. Marcelin, M. Rosenzwajg, D. Klatzmann et al., Neutrophil-platelet and monocyte-platelet aggregates in COVID-19 patients. Thromb. Haemostasis 120, 1733–1735 (2020)
S. Chen, J. Zhu, J. Xue, X. Wang, P. Jing, L. Zhou, Y. Cui, T. Wang, X. Gong, S. Lu, M. Long, Numerical simulation of flow characteristics in a permeable liver sinusoid with leukocytes. Biophys. J. 121, 4666–4678 (2022)
H. Li, K. Sampani, X. Zheng, D. Papageorgiou, A. Yazdani, M. Bernabeu, G.E. Karniadakis, J. Sun, Predictive modelling of thrombus formation in diabetic retinal microaneurysms. R. Soc. Open Sci. 7, 201102 (2020)
X. Zheng, A. Yazdani, H. Li, J. Humphrey, G.E. Karniadakis, A three-dimensional phase-field model for multiscale modeling of thrombus biomechanics in blood vessels. PLOS Comput. Biol. 16, 1007709 (2020)
H. Li, Y. Deng, K. Sampani, S. Cai, Z. Li, J.K. Sun, G.E. Karniadakis, Computational investigation of blood cell transport in retinal microaneurysms. PLOS Comput. Biol. 18, 1009728 (2022)
L. Xiao, J. Chu, C. Lin, K. Zhang, S. Chen, L. Yang, Simulation of a tumor cell flowing through a symmetric bifurcated microvessel. Biomech. Model. Mechanobiol. 22, 297–308 (2023)
Y. Deng, D.P. Papageorgiou, X. Li, N. Perakakis, C.S. Mantzoros, M. Dao, G.E. Karniadakis, Quantifying fibrinogen-dependent aggregation of red blood cells in type 2 diabetes mellitus. Biophys. J. 119, 900–912 (2020)
E. Javadi, Y. Deng, G.E. Karniadakis, S. Jamali, In silico biophysics and hemorheology of blood hyperviscosity syndrome. Biophys. J. 120, 2723–2733 (2021)
E. Javadi, H. Li, A.D. Gallastegi, G.H. Frydman, S. Jamali, G.E. Karniadakis, Circulating cell clusters aggravate the hemorheological abnormalities in COVID-19. Biophys. J. 121, 3309–3319 (2022)
L. Li, S. Wang, K. Han, X. Qi, S. Ma, L. Li, J. Yin, D. Li, X. Li, J. Qian, Quantifying shear-induced margination and adhesion of platelets in microvascular blood flow. J. Mol. Biol. 435, 167824 (2023)
H. Li, Y. Deng, Z. Li, A. Dorken Gallastegi, C.S. Mantzoros, G.H. Frydman, G.E. Karniadakis, Multiphysics and multiscale modeling of microthrombosis in COVID-19. PLOS Comput. Biol. 18, 1009892 (2022)
I.V. Pivkin, G.E. Karniadakis, Accurate coarse-grained modeling of red blood cells. Phys. Rev. Lett. 101, 118105 (2008)
D.A. Fedosov, B. Caswell, G.E. Karniadakis, Systematic coarse-graining of spectrin-level red blood cell models. Comput. Methods Appl. Mech. Eng. 199, 1937–1948 (2010)
Z. Peng, X. Li, I.V. Pivkin, M. Dao, G.E. Karniadakis, S. Suresh, Lipid bilayer and cytoskeletal interactions in a red blood cell. Proc. Natl. Acad. Sci. USA 110, 13356–13361 (2013)
S. Ma, S. Wang, X. Qi, K. Han, X. Jin, Z. Li, G. Hu, X. Li, Multiscale computational framework for predicting viscoelasticity of red blood cells in aging and mechanical fatigue. Comput. Methods Appl. Mech. Eng. 391, 114535 (2022)
P. Hoogerbrugge, J. Koelman, Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics. Europhys. Lett. 19, 155 (1992)
H. Lei, B. Caswell, G.E. Karniadakis, Direct construction of mesoscopic models from microscopic simulations. Phys. Rev. E 81, 026704 (2010)
Z. Li, X. Bian, B. Caswell, G.E. Karniadakis, Construction of dissipative particle dynamics models for complex fluids via the Mori-Zwanzig formulation. Soft Matter 10, 8659–8672 (2014)
M.L. Klein, W. Shinoda, Large-scale molecular dynamics simulations of self-assembling systems. Science 321, 798–800 (2008)
H. Li, G. Lykotrafitis, A coarse-grain molecular dynamics model for sickle hemoglobin fibers. J. Mech. Behav. Biomed. Mater. 4, 162–173 (2011)
L. Lu, H. Li, X. Bian, X. Li, G.E. Karniadakis, Mesoscopic adaptive resolution scheme toward understanding of interactions between sickle cell fibers. Biophys. J. 113, 48–59 (2017)
L. Lu, Y. Deng, X. Li, H. Li, G.E. Karniadakis, Understanding the twisted structure of amyloid fibrils via molecular simulations. J. Phys. Chem. B 122, 11302–11310 (2018)
Z. Adamczyk, J. Barbasz, M. Ciesla, Mechanisms of fibrinogen adsorption at solid substrates. Langmuir 27, 6868–6878 (2011)
X. Li, B. Caswell, G.E. Karniadakis, Effect of chain chirality on the self-assembly of sickle hemoglobin. Biophys. J. 103, 1130–1140 (2012)
E. Caspary, R. Kekwick, Some physicochemical properties of human fibrinogen. Biochem. J. 67, 41 (1957)
W.E. Fowler, H.P. Erickson, Trinodular structure of fibrinogen: confirmation by both shadowing and negative stain electron microscopy. J. Mol. Biol. 134, 241–249 (1979)
D.A. Fedosov, W. Pan, B. Caswell, G. Gompper, G.E. Karniadakis, Predicting human blood viscosity in silico. Proc. Natl. Acad. Sci. USA 108, 11772–11777 (2011)
M. Dembo, D. Torney, K. Saxman, D. Hammer, The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc. Natl. Acad. Sci. USA 234, 55–83 (1988)
D.A. Fedosov, B. Caswell, S. Suresh, G.E. Karniadakis, Quantifying the biophysical characteristics of Plasmodium-falciparum-parasitized red blood cells in microcirculation. Proc. Natl. Acad. Sci. USA 108, 35–39 (2011)
J. Kim, N.E. Hudson, T.A. Springer, Force-induced on-rate switching and modulation by mutations in gain-of-function von willebrand diseases. Proc. Natl. Acad. Sci. USA 112, 4648–4653 (2015)
A. Yazdani, Y. Deng, H. Li, E. Javadi, Z. Li, S. Jamali, C. Lin, J.D. Humphrey, C.S. Mantzoros, G.E. Karniadakis, Integrating blood cell mechanics, platelet adhesive dynamics and coagulation cascade for modelling thrombus formation in normal and diabetic blood. J. R. Soc. Interface 18, 20200834 (2021)
A.D. Truong, S.C. Auld, N.A. Barker, S. Friend, A.T. Wynn, J. Cobb, R.M. Sniecinski, C.-L. Tanksley, D.M. Polly, M. Gaddh et al., Therapeutic plasma exchange for COVID-19-associated hyperviscosity. Transfusion 61, 1029–1034 (2021)
M.D. Berger, A.D. Heini, K. Seipel, B. Mueller, A. Angelillo-Scherrer, T. Pabst, Increased fibrinogen levels at diagnosis are associated with adverse outcome in patients with acute myeloid leukemia. Hematol. Oncol. 35, 789–796 (2017)
J. Backer, C. Lowe, H. Hoefsloot, P. Iedema, Poiseuille flow to measure the viscosity of particle model fluids. J. Chem Phys. 122, 154503 (2005)
H. Li, G. Lykotrafitis, Two-component coarse-grained molecular-dynamics model for the human erythrocyte membrane. Biophys. J. 102, 75–84 (2012)
H. Li, G. Lykotrafitis, Erythrocyte membrane model with explicit description of the lipid bilayer and the spectrin network. Biophys. J. 107, 642–653 (2014)
Y. Zhang, C. Huang, S. Kim, M. Golkaram, M.W.A. Dixon, L. Tilley, J. Li, S. Zhang, S. Suresh, Multiple stiffening effects of nanoscale knobs on human red blood cells infected with Plasmodium falciparum malaria parasite. Proc. Natl. Acad. Sci. USA 112, 6068–6073 (2015)
H. Li, Y. Zhang, V. Ha, G. Lykotrafitis, Modeling of band-3 protein diffusion in the normal and defective red blood cell membrane. Soft Matter 12, 3643–3653 (2016)
H. Li, J. Yang, T.T. Chu, R. Naidu, L. Lu, R. Chandramohanadas, M. Dao, G.E. Karniadakis, Cytoskeleton remodeling induces membrane stiffness and stability changes of maturing reticulocytes. Biophys. J. 114, 2014–2023 (2018)
Y. Deng, D.P. Papageorgiou, H.-Y. Chang, S.Z. Abidi, X. Li, M. Dao, G.E. Karniadakis, Quantifying shear-induced deformation and detachment of individual adherent sickle red blood cells. Biophys. J. 116, 360–371 (2019)
Y. Huo, G.S. Kassab, The scaling of blood flow resistance: from a single vessel to the entire distal tree. Biophys. J. 96, 339–346 (2009)
G.S. Kassab, Scaling laws of vascular trees: of form and function. Am. J. Physiol. Heart Circ. Physiol. 290, 894–903 (2006)
D. Xiu, G.E. Karniadakis, Modeling uncertainty in flow simulations via generalized polynomial chaos. J. Comput. Phys. 187, 137–167 (2003)
T.-R. Lee, M.S. Greene, Z. Jiang, A.M. Kopacz, P. Decuzzi, W. Chen, W.K. Liu, Quantifying uncertainties in the microvascular transport of nanoparticles. Biomech. Model. Mechanobiol. 13, 515–526 (2014)
S. Sankaran, H.J. Kim, G. Choi, C.A. Taylor, Uncertainty quantification in coronary blood flow simulations: impact of geometry, boundary conditions and blood viscosity. J. Biomech. 49, 2540–2547 (2016)
G. Bertaglia, V. Caleffi, L. Pareschi, A. Valiani, Uncertainty quantification of viscoelastic parameters in arterial hemodynamics with the a-FSI blood flow model. J. Comput. Phys. 430, 110102 (2021)
Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (no. LY22A020004), and the Fundamental Research Funds for the Central Universities (no. 226-2022-00125). Simulations were conducted at the Beijing Super Cloud Computing Center (BLSC).
Author information
Authors and Affiliations
Contributions
KH: formal analysis, investigation, methodology, validation, writing—original draft, writing—review and editing, visualization. WZ: formal analysis, writing— review and editing. SM: formal analysis, methodology, writing—review and editing. SW: formal analysis, methodology, writing—review and editing. XQ: formal analysis, methodology, writing—review and editing. LG: conceptualization, supervision, writing—review and editing. XL: conceptualization, formal analysis, supervision, writing—original draft, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 7179 kb)
Supplementary file3 (MP4 6130 kb)
Supplementary file4 (MP4 4895 kb)
Supplementary file5 (MP4 5544 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, K., Zhou, W., Ma, S. et al. A computational study of fibrinogen-induced alteration in microvascular blood flow in COVID-19. Eur. Phys. J. Spec. Top. 232, 2761–2772 (2023). https://doi.org/10.1140/epjs/s11734-023-00901-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-023-00901-w