Abstract
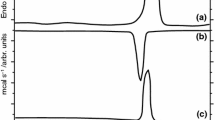
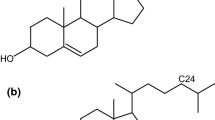
The amphiphilic phospholipids have two hydrophobic fatty acids separated by charged hydrophilic head groups. They can form bilayers with the interior fatty acids oriented parallel to each other and with the phospholipid heads facing out in contact with water. In order to avoid the complexity of the natural membranes, these bilayers are used as model membranes. At room temperature the model membranes are characterized by a high degree of order in the gel state and, at higher temperature they pass, by a sudden process, in a less ordered state of liquid crystalline type. This phenomenon depends on the phospholipid type as well as on the nature of the possible impurities and it is conditioned by temperature. Our studies were made for dipalmitoylphophatidylcholine model membranes in which some drugs as gramicidin S and nifedipine were added to establish the drug influence on the main phase transition temperature. Gramicidin S decreases the main phase transition temperature, while nifedipine increases the temperature of the main phase transition of DPPC model membranes. Research of the model membranes near the main phase transition can facilitate the understanding of the destabilization by the drug action mechanism of the biological membranes.







Similar content being viewed by others
Data availability
All data generated and analyzed during this study are available from the corresponding author on reasonable request.
References
M. Jackson, H.H. Mantsch, Biomembrane structure from FTIR spectroscopy. Spectrochim. Acta Rev. 15, 53–69 (1993)
M.K. Jain, Introduction to biological membranes, 2nd edn. (Wiley, New York, 1988)
A. Blume, W. Huebner, G. Messner, Fourier transform infrared spectroscopy of 13C=O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry 27(21), 8239–8249 (1988). https://doi.org/10.1021/bi00421a038
E. Egberts, S.-J. Marrink, H.J.C. Berendsen, Molecular dynamics simulation of a phospholipid membrane. Eur. Biophys. J. 22(6), 423–436 (1994). https://doi.org/10.1007/BF00180163
B.G. Akinoglu, M. Gheith, F. Severcan, Thermodynamics study of gramicidin S and dipalmitoylphosphatidylcholine model membrane interactions based on the FTIR spectroscopy. J. Mol. Struct. 565–566, 281–285 (2001). https://doi.org/10.1016/S0022-2860(01)00503-8
F. Severcan, C. Agheorghiesei, D.O. Dorohoi, Temperature dependence of the phospholipids bilayers stability, studied by FTIR spectroscopy. Rev. Chim. Buc. 59(3), 356–359 (2008). https://doi.org/10.37358/RC.08.3.1762
C. Stan, C.P. Cristescu, F. Severcan, D. Dorohoi, Effect of gramicidin S on the dipalmitoylphosphatidylglycerol thermotropic phase transition in DPPG/GS systems: A mathematical approach. Mol. Cryst. Liq. Cryst. 457, 27–41 (2006). https://doi.org/10.1080/15421400500447116
G.F. Gause, M.G. Brazhnikova, Gramicidin S and its use in the treatment of infected wounds. Nature 154, 703 (1944). https://doi.org/10.1038/154703a0
N. Izumiya, T. Kato, H. Aoyagi, M. Waki, M. Kondo, Synthetic aspects of biologically active cyclic peptides – gramicidin S and tyrocidines (Halsted Press Book, New York, 1979)
E.J. Prenner, R.N.A.H. Lewis, L.H. Kondejewski, R.S. Hodges, R.N. McElhaney, Differential scanning calorimetric study of the effect of the antimicrobial peptide gramicidin S on the thermotropic phase behavior of phosphatidylcholine, phosphatidylethanolamine and phosphatidylglycerol lipid bilayer membranes. Biochim. Biophys. Acta Biomembr. 1417(2), 211–223 (1999). https://doi.org/10.1016/S0005-2736(99)00004-8
E.J. Prenner, R.N.A.H. Lewis, R.N. McElhaney, The interaction of the antimicrobial peptide gramicidin S with lipid bilayer model and biological membranes. Biochim. Biophys. Acta Biomembr. 1462(1–2), 201–221 (1999). https://doi.org/10.1016/S0005-2736(99)00207-2
A.L. Lehninger, Biochemistry, 2nd edn. (Worth Publishers, New York, 1975)
K. Lohner, E.J. Prenner, Differential scanning calorimetry and X-ray diffraction study of the specificity of the interaction of antimicrobial peptides with membrane-mimetic systems. Biochim. Biophys. Acta Biomembr. 1462(1–2), 141–156 (1999). https://doi.org/10.1016/S0005-2736(99)00204-7
T. Katsu, H. Kobayashi, T. Hirota, Y. Fujita, K. Sato, U. Nagay, Structure-activity relationship of gramicidin S analogues on membrane permeability. Biochim. Biophys. Acta Biomembr. 899(2), 159–170 (1987). https://doi.org/10.1016/0005-2736(87)90396-8
F. Severcan, H.O. Durmus, F. Eker, B.G. Akinogluy, P.I. Haris, Vitamin D2 modulates melittin-membrane interactions. Talanta 53(1), 205–211 (2000). https://doi.org/10.1016/S0039-9140(00)00453-7
W. Vater, G. Kroneberg, F. Hoffmeister, H. Saller, K. Meng, A. Oberdorf, W. Puls, K. Schlossmann, K. Stoepel, Pharmacology of 4-(2’-nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid dimethyl ester (Nifedipine, BAY a 1040) (in German). Arzneimittelforschung. 22(1), 1–14 (1972)
T.M. Curtis, C.N. Scholfield, Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J. Physiol 532(3), 609–623 (2001). https://doi.org/10.1111/j.1469-7793.2001.0609e.x
D.R. Sliskovic, Cardiovascular Drugs, in Drug Discovery: Practices, Processes, and Perspectives, ed. by J.J. Li, E.J. Corey (John Wiley & Sons, Hoboken, 2013) p.141–204
G. Gaviraghi, Case Study of Lacidipine in the Research of New Calcium Antagonists, in Analog-based Drug Discovery. ed. by J. Fischer, C.R. Ganellin (Wiley-VCH, Weinheim, 2006), pp.181–192
J.M. Luther, Is there a new dawn for selective mineralocorticoid receptor antagonism? Curr. Opin. Nephrol. Hypertens. 23(5), 456–461 (2014). https://doi.org/10.1097/MNH.00000000000000051
G.M. Petrov, A simple algorithm for spectral line deconvolution. J. Quant. Spectrosc. Radiat. Transf. 72(3), 281–287 (2002). https://doi.org/10.1016/S0022-4073(01)00125-X
Spartan’14 for Windows, Macintosh and Linux, Tutorial and User’s Guide, January 10, 2014, available online at http://downloads.wavefun.com/Spartan14Manual.pdf
W.J. Hehre, A guide to molecular mechanics and quantum chemical calculations (Wavefunction Inc., Irvine, 2003)
J.R.L. Arrondo, F.M. Goñi, J.M. Macarulla, Infrared spectroscopy of phosphatidylcholines in aqueous suspension. A study of the phosphate group vibrations. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 794(1), 165–168 (1984). https://doi.org/10.1016/0005-2760(84)90310-2
L.C. Stewart, M. Kates, P.W. Yang, H.H. Mantsch, Intra- and inter-molecular hydrogen bonding in diphytanylglycerol phospholipids: an infrared spectroscopic investigation. Biochem. Cell Biol. 68(1), 266–273 (1990). https://doi.org/10.1139/o90-037
M. Avram, G.D. Mateescu, Infrared Spectroscopy. Applications in Organic Chemistry (in Romanian) (Ed. Tehnică, București, 1966)
A.H. de Vries, A.E. Mark, S.J. Marrink, Molecular dynamics simulation of the spontaneous formation of a small DPPC vesicle in water in atomistic detail. J. Am. Chem. Soc. 126(14), 4488–4489 (2004). https://doi.org/10.1021/ja0398417
A. Lenz, L. Ojamäe, Theoretical IR spectra for water clusters (H2O)n (n = 6–22, 28, 30) and identification of spectral contributions from different H-bond conformations in gaseous and liquid water. J. Phys. Chem. A 110(50), 13388–13393 (2006). https://doi.org/10.1021/jp066372x
F. Korkmaz, F. Severcan, M. Aflori, D.O. Dorohoi, Temperature influence on the dyphalmitoylphosphatidylcholyne——model membranes studied by FTIR. Dig. J. Nanomater. Biostructures 3(2), 55–61 (2008)
J.J. López Cascales, T.F. Otero, B.D. Smith, C. González, M. Márquez, Model of an asymmetric DPPC/DPPS membrane: effect of asymmetry of the lipid properties. A molecular dynamics simulation study. J. Phys. Chem. B 110(5), 2358–2363 (2006). https://doi.org/10.1021/jp0562680
I. Noda, Y. Ozaki, Two-dimensional Correlation Spectroscopy. Applications in Vibrational and Optical Spectroscopy (John Wiley & Sons, Chichester, 2004)
F. Severcan, D.O. Dorohoi, FTIR studies of temperature influence on the DPPG model membrane. J. Mol. Struct. 887(1–3), 117–121 (2008). https://doi.org/10.1016/j.molstruc.2008.02.039
Funding
The study was funded by Romanian Ministry of Research, Inovation and Digitization (Grant no. 11PFE/30.12.2021).
Author information
Authors and Affiliations
Contributions
The authors equally contributed to all activities related to this article.
Corresponding author
Ethics declarations
Conflict of interest
Authors are thankful to Romanian Ministry of Research, Innovation and Digitization, within Program 1—Development of the national RD system, Subprogram 1.2—Institutional Performance—RDI excellence funding projects, Contract no. 11PFE/30.12.2021, for financial support.
Additional information
IMA10 - Interfacial Fluid Dynamics and Processes. Guest editors: Rodica Borcia, Sebastian Popescu, Ion Dan Borcia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dimitriu, D.G., Dorohoi, D.O. Drug and temperature influence on the gel state stability of the phospholipid membranes. Eur. Phys. J. Spec. Top. 232, 427–433 (2023). https://doi.org/10.1140/epjs/s11734-023-00784-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-023-00784-x