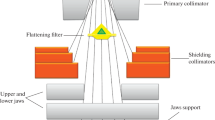
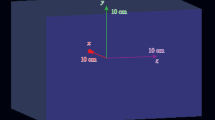
Abstract
Spatially fractionated radiation therapy (SFRT) is an approach that spares healthy tissue compared to conventional radiation therapy. Light ions have also more advantages over heavy charged particles and X-ray beams including physical and radiobiological aspects. The composition of SFRT, particularly minibeam radiation therapy (MBRT), with these privileges could improve the therapeutic index. Monte Carlo simulations were performed by Geant4 (Geant4-11.0.1) to evaluate the radiation of broad beam, single, and arrays of proton and light ion minibeams in a water phantom. Several minibeam sizes and center-to-center (ctc) distances were selected. The contribution of different secondary species, peak and valley doses, peak-to-valley dose ratio (PVDR), and Bragg peak-to-entrance dose ratio (BEDR) was studied. Light ion minibeams have higher PVDR in normal tissues, more BEDR, and even were broadened more slowly compared to protons in same ctcs. Reduced lateral scattering for heavier ions than protons creates sharper peaks and lower valley doses. A higher ctc (3.5 mm) can enhance normal tissue-sparing due to its higher PVDR and cause lower contribution of secondary fragments, but dose conformity is more complicated in the target region for heavier ions. This non-uniformity can be reduced when ctc decreases (1.2 mm), while perfect normal tissue-sparing could not be achieved. Although the contribution of nuclear products is enhanced with atomic number of incident ions, dominant dose deposition occurs at deeper depths in valleys. These results highlight that light ions fulfill advantageous dose profiles and might be good candidates for MBRT.












Similar content being viewed by others
Data Availability Statement
The data sets generated during the present study are available from the corresponding author upon reasonable request. The manuscript has associated data in a data repository.
References
M. Sammer, C. Greubel, S. Girst, G. Dollinger, Optimization of beam arrangements in proton minibeam radiotherapy by cell survival simulations. Med. Phys. 44(11), 6096–6104 (2017). https://doi.org/10.1002/mp.12566
L. De Marzi, C. Nauraye, P. Lansonneur et al., Spatial fractionation of the dose in proton therapy: proton minibeam radiation therapy. Cancer Radiother. 23(6–7), 677–681 (2019). https://doi.org/10.1016/j.canrad.2019.08.001
S. Keshmiri, S. Brocard, R. Serduc, J.F. Adam, A high-resolution dose calculation engine for X-ray microbeams radiation therapy. Med. Phys. 49(6), 3999–4017 (2022). https://doi.org/10.1002/mp.15637
S. Bartzsch, S. Corde, J.C. Crosbie, L. Day et al., Technical advances in X-ray microbeam radiation therapy. Phys. Med. Biol. 65(2), 02TR01 (2020). https://doi.org/10.1088/1361-6560/ab5507
R. Ortiz, R. Belshi, L. De Marzi, Y. Prezado, Proton minibeam radiation therapy for treating metastases: a treatment plan study. Med. Phys. 50(4), 1–11 (2023). https://doi.org/10.1002/mp.16203
T.R. Johnson, A.M. Bassil, N.T. Williams et al., An investigation of kV mini-GRID spatially fractionated radiation therapy: dosimetry and preclinical trial. Phys. Med. Biol. 67(4), 045017 (2022). https://doi.org/10.1088/1361-6560/ac508c
X. Wu, M.M. Ahmed, J. Wright et al., On modern technical approaches of three-dimensional high-dose lattice radiotherapy (LRT). Cureus 2(3), e9 (2010). https://doi.org/10.7759/cureus.9
F.A. Dilmanian, T.M. Button, G. Le Duc et al., Response of rat intracranial 9L gliosarcoma to microbeam radiation therapy. Neuro Oncol. 4(1), 26–38 (2002). https://doi.org/10.1093/neuonc/4.1.26
Y.R. Lawrence, X.A. Li, I. El Naqa et al., Radiation dose–volume effects in the brain. Int. J. Radiat. Oncol. Biol. Phys. 76(3), S20–S27 (2010). https://doi.org/10.1016/j.ijrobp.2009.02.091
T. Schneider, Technical aspects of proton minibeam radiation therapy: minibeam generation and delivery. Phys. Med. 100, 64–71 (2022). https://doi.org/10.1016/j.ejmp.2022.06.010
Y. Prezado, M. Dos Santos, W. Gonzalez et al., Transfer of minibeam radiation therapy into a cost-effective equipment for radiobiological studies: a proof of concept. Sci. Rep. 7(1), 17295 (2017). https://doi.org/10.1038/s41598-017-17543-3
T. Schneider, D. Malaise, F. Pouzoulet, Y. Prezado, Orthovoltage X-ray minibeam radiation therapy for the treatment of ocular tumours—an in silico evaluation. Cancers 15(3), 679 (2023). https://doi.org/10.3390/cancers15030679
I. Momot, O. Kovalchuk, O. Okhrimenko et al., Shaping and monitoring of the mini-beam structures for the spatially fractionated hadron radiation therapy. Nucl. Phys. At. Energy 17(1), 92–97 (2016). https://doi.org/10.15407/jnpae2016.01.092
U. Amaldi, S. Braccini, Present challenges in hadrontherapy techniques. Eur. Phys. J. Plus 126(67), 70 (2011). https://doi.org/10.1140/epjp/i2011-11067-y
Y. Prezado, G.R. Fois, Proton-minibeam radiation therapy: a proof of concept. Med. Phys. 40(3), 031712 (2013). https://doi.org/10.1118/1.4791648
O. Zlobinskaya, S. Girst, C. Greubel et al., Reduced side effects by proton microchannel radiotherapy: study in a human skin model. Radiat. Environ. Biophys. 52, 123–133 (2013). https://doi.org/10.1007/s00411-012-0450-9
D. Bolst, S. Guatelli, L. Tran et al., Validation of Geant4 for silicon microdosimetry in heavy ion therapy. Phys. Med. Biol. 65(4), 045014 (2020). https://doi.org/10.1088/1361-6560/ab586a
T. Tsubouchi, T. Henry, A. Ureba et al., Quantitative evaluation of potential irradiation geometries for carbon-ion beam grid therapy. Med. Phys. 45(3), 1210–1221 (2018). https://doi.org/10.1002/mp.12749
I. Martínez-Rovira, W. González, S. Brons, Y. Prezado, Carbon and oxygen minibeam radiation therapy: an experimental dosimetric evaluation. Med. Phys. 44(8), 4223–4229 (2017). https://doi.org/10.1002/mp.12383
C. Guardiola, Y. Prezado, High-energy charged particles for spatially fractionated radiation therapy. Front. Phys. 8, 299 (2020). https://doi.org/10.3389/fphy.2020.00299
I. Martinez-Rovira, S. Brons, Y. Prezado, Hadron minibeam radiation therapy: feasibility study at the Heidelberg Ion-Beam Therapy Center (HIT). Radiother. Oncol. 118, S70–S71 (2016). https://doi.org/10.1016/S0167-8140(16)30144-X
W. González, Y. Prezado, Spatial fractionation of the dose in heavy ions therapy: an optimization study. Med. Phys. 45(6), 2620–2627 (2018). https://doi.org/10.1002/mp.12902
Y. Prezado, R. Hirayama, N. Matsufuji et al., A potential renewed use of very heavy ions for therapy: neon minibeam radiation therapy. Cancers 13(6), 1356 (2021). https://doi.org/10.3390/cancers13061356
T. Tessonnier, A. Mairani, W. Chen et al., Proton and helium ion radiotherapy for meningioma tumors: a Monte Carlo-based treatment planning comparison. Radiother. Oncol. 13(2), 1–10 (2018). https://doi.org/10.1186/s13014-017-0944-3
T. Schneider, A. Patriarca, Y. Prezado, Improving the dose distributions in minibeam radiation therapy: helium ions vs protons. Med. Phys. 46(8), 3640–3648 (2019). https://doi.org/10.1002/mp.13646
F.A. Dilmanian, J.G. Eley, S. Krishnan, Minibeam therapy with protons and light ions: physical feasibility and potential to reduce radiation side effects and to facilitate hypofractionation. Int. J. Radiat. Oncol. Biol. Phys. 92(2), 469–474 (2015). https://doi.org/10.1016/j.ijrobp.2015.01.018
S. Agostinelli, J. Allison, K.A. Amako et al., GEANT4—a simulation toolkit. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 506(3), 250–303 (2003). https://doi.org/10.1016/S0168-9002(03)01368-8
P. Arce, D. Bolst, M.C. Bordage et al., Report on G4-Med, a Geant4 benchmarking system for medical physics applications developed by the Geant4 Medical Simulation Benchmarking Group. Med. Phys. 48(1), 19–56 (2021). https://doi.org/10.1002/mp.14226
C. Guardiola, C. Peucelle, Y. Prezado, Optimization of the mechanical collimation for minibeam generation in proton minibeam radiation therapy. Med. Phys. 44(4), 1470–1478 (2017). https://doi.org/10.1002/mp.12131
W. González, C. Peucelle, Y. Prezado, Theoretical dosimetric evaluation of carbon and oxygen minibeam radiation therapy. Med. Phys. 44(5), 1921–1929 (2017). https://doi.org/10.1002/mp.12175
C. Peucelle, I. Martínez-Rovira, Y. Prezado, Spatial fractionation of the dose using neon and heavier ions: a Monte Carlo study. Med. Phys. 42(10), 5928–5936 (2015). https://doi.org/10.1118/1.4930960
Y. Prezado, S. Thengumpallil, M. Renier, A. Bravin, X-ray energy optimization in minibeam radiation therapy. Med. Phys. 36(11), 4897–4902 (2009). https://doi.org/10.1118/1.3232000
Y. Prezado, G. Jouvion, C. Guardiola et al., Tumor control in RG2 glioma-bearing rats: a comparison between proton minibeam therapy and standard proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 104(2), 266–271 (2019). https://doi.org/10.1016/j.ijrobp.2019.01.080
Funding
There is no funding available for the publication of this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajabnejad, M., Ghasemizad, A. & Zabihi, A. Dosimetric evaluation of light ion beams for spatially fractionated radiation therapy: a Geant4 Monte Carlo study. Eur. Phys. J. Plus 139, 365 (2024). https://doi.org/10.1140/epjp/s13360-024-05162-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-024-05162-7