Abstract
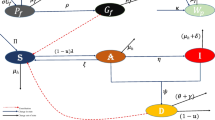
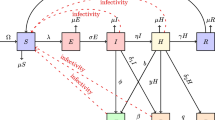
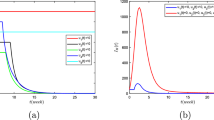
Lymphatic filariasis represents the primary cause of long-term, permanent disability, and dysfunction in the human immune system. In this study, we have devised and assessed deterministic and continuous-time Markov chain (CTMC) stochastic models to gain insights into the dynamics of lymphatic filariasis and approximate the probabilities of disease extinction or outbreak. The CTMC stochastic model is an adapted version of the existing deterministic model that accounts for uncertainties and variations in disease transmission dynamics. The findings from the deterministic model indicate that disease extinction is possible when \({\mathcal {R}}_0 < 1\), while an outbreak is likely when \({\mathcal {R}}_0 > 1\). Further examination of the deterministic model emphasizes the significant role of asymptomatic individuals in the transmission of lymphatic filariasis. To estimate the probabilities of disease extinction or outbreak, we employed multitype branching processes and numerical simulations. The results demonstrate that lymphatic filariasis outbreaks are more probable when microfilariae parasites are introduced by exposed humans, asymptomatic humans, acutely infected humans, exposed mosquitoes, or infectious mosquitoes. Conversely, the disease is more likely to be eradicated if it originates from chronically infected humans. Utilizing stochastic methods provides a more authentic portrayal of how lymphatic filariasis spreads, granting a better understanding of the spectrum of potential results and their related probabilities. Therefore, stochastic CTMC models become indispensable for generating reliable forecasts and well-informed choices in situations where deterministic models might oversimplify or inaccurately depict the inherent unpredictability.









Similar content being viewed by others
Data Availability Statement
The data used for this study are included in this paper.
References
S. Surtani, J. Kailashiya, M.A. Ansari, D. Dash, A.K. Yadav, A. Kumar, Platelet functions in lymphatic filariasis patients. Microvasc. Res. 152, 104642 (2024)
WHO, Lymphatic filariasis: World Health Organisation (2022). https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis/
D. Addiss, The 6th meeting of the global alliance to eliminate lymphatic filariasis: a half-time review of lymphatic filariasis elimination and its integration with the control of other neglected tropical diseases(2010)
S. Lustigman, R.K. Prichard, A. Gazzinelli, W.N. Grant, B.A. Boatin, J.S. McCarthy, M.-G. Basáñez, A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl. Trop. Dis. 6(4), e1582 (2012)
CDC, Parasites—lymphatic filariasis: Centers for Diseases Control and Prevention. https://www.cdc.gov/parasites/lymphaticfilariasis/epi.html (2022)
Centers for Disease Control and Prevention, Lymphatic Filariasis. https://www.cdc.gov/dpdx/lymphaticfilariasis/index.htm (2019). Accessed 26 Jan 2023
J.C. Castillo, S.E. Reynolds, I. Eleftherianos, Insect immune responses to nematode parasites. Trends Parasitol. 27(12), 537–547 (2011)
P. Fischer, S.M. Erickson, K. Fischer, J.F. Fuchs, R.U. Rao, B.M. Christensen, G.J. Weil, Persistence of Brugia malayi DNA in vector and non-vector mosquitoes: implications for xenomonitoring and transmission monitoring of lymphatic filariasis. Am. J. Trop. Med. Hyg. 76(3), 502 (2007)
A.A. Hamza, M.M. Dogara, J.B. Balogun, A.I. Omotayo, K.A. Adeniyi, A.S. Abubakar, A.A. Hafiz, S.S. Abubakar, Entomological surveillance reveals transmission of malaria but not lymphatic filariasis in two communities in north-west nigeria. Parasitol. Res. 123(1), 26 (2024)
M.J. Taylor, A. Hoerauf, M. Bockarie, Lymphatic filariasis and onchocerciasis. The Lancet 376(9747), 1175–1185 (2010)
A. Singal, K. Bisherwal, Integrative medicine and self-care in the treatment of lymphatic filariasis-associated lymphoedema: an effective strategy! Br. J. Dermatol. 190(1), 8–9 (2024)
S. Michael, N. Mbwambo, H. Mruttu, M. Dotto, C. Ndomba, M.d. Silva, F. Makusaro, S. Nandonde, J. Crispin, B.I. Shapiro, et al., Tanzania livestock master plan (2018)
M.A. Stephano, M.M. Mayengo, J.I. Irunde, D. Kuznetsov, Sensitivity analysis and parameters estimation for the transmission of lymphatic filariasis. Heliyon (2023)
J. Paul, Blood and lymphatic infections, in Disease Causing Microbes (Springer, 2024), pp. 247–314
D. Buonfrate, A. Montresor, Z. Bisoffi, F. Tamarozzi, D. Bisanzio, Progress towards the implementation of control programmes for strongyloidiasis in endemic areas: estimation of number of adults in need of ivermectin for strongyloidiasis. Philos. Trans. R. Soc. B 379(1894), 20220433 (2024)
C. Naing, M.A. Whittaker, W.S. Tung, H. Aung, J.W. Mak, Prevalence of zoonotic (Brugian) filariasis in Asia: a proportional meta-analysis. Acta Trop. 249, 107049 (2024)
P.M. Mwamtobe, S.M. Simelane, S. Abelman, J.M. Tchuenche, Mathematical analysis of a lymphatic filariasis model with quarantine and treatment. BMC Public Health 17(1), 1–13 (2017)
P. Jambulingam, S. Subramanian, S. De Vlas, C. Vinubala, W. Stolk, Mathematical modelling of lymphatic filariasis elimination programmes in India: required duration of mass drug administration and post-treatment level of infection indicators. Parasites Vectors 9(1), 1–18 (2016)
E. Michael, M.N. Malecela-Lazaro, P.E. Simonsen, E.M. Pedersen, G. Barker, A. Kumar, J.W. Kazura, Mathematical modelling and the control of lymphatic filariasis. Lancet. Infect. Dis. 4(4), 223–234 (2004)
S. Swaminathan, P.P. Subash, R. Rengachari, K. Kaliannagounder, D.K. Pradeep, Mathematical models for lymphatic filariasis transmission and control: challenges and prospects. Parasites Vectors 1(1), 1–9 (2008)
W.A. Stolk, C. Stone, S.J. de Vlas, Modelling lymphatic filariasis transmission and control: modelling frameworks, lessons learned and future directions. Adv. Parasitol. 87, 249–291 (2015)
A. Alshehri, Z. Shah, R. Jan, Mathematical study of the dynamics of lymphatic filariasis infection via fractional-calculus. Eur. Phys. J. Plus 138(3), 1–15 (2023)
E.A. Cromwell, C.A. Schmidt, K.T. Kwong, D.M. Pigott, D. Mupfasoni, G. Biswas, S. Shirude, E. Hill, K.M. Donkers, A. Abdoli et al., The global distribution of lymphatic filariasis, 2000–18: a geospatial analysis. Lancet Glob. Health 8(9), e1186–e1194 (2020)
L.J. Allen, An introduction to stochastic epidemic models, in Mathematical Epidemiology (Springer, 2008), pp. 81–130
L.J. Allen, An Introduction to Stochastic Processes with Applications to Biology (CRC Press, Boca Raton, 2010)
L.J. Allen, G.E. Lahodny Jr., Extinction thresholds in deterministic and stochastic epidemic models. J. Biol. Dyn. 6(2), 590–611 (2012)
L.J. Allen, P. van den Driessche, Relations between deterministic and stochastic thresholds for disease extinction in continuous-and discrete-time infectious disease models. Math. Biosci. 243(1), 99–108 (2013)
L.J. Allen, A primer on stochastic epidemic models: formulation, numerical simulation, and analysis. Infect. Dis. Model. 2(2), 128–142 (2017)
M. Maliyoni, Probability of disease extinction or outbreak in a stochastic epidemic model for west Nile virus dynamics in birds. Acta Biotheor. 69, 1–26 (2020)
M. Maliyoni, F. Chirove, H.D. Gaff, K.S. Govinder, A stochastic tick-borne disease model: exploring the probability of pathogen persistence. Bull. Math. Biol. 79(9), 1999–2021 (2017)
M.A. Stephano, J.I. Irunde, J.A. Mwasunda, C.S. Chacha, A continuous time Markov chain model for the dynamics of bovine tuberculosis in humans and cattle. Ricerche di Matematica, 1–27 (2022)
S. Swaminathan, Modelling Lymphatic Filariasis: Transmission and Control (2004)
V. Lakshmikantham, S. Leela, A.A. Martynyuk, Stability Analysis of Nonlinear Systems (Springer, Berlin, 1989)
K. Dietz, The estimation of the basic reproduction number for infectious diseases. Stat. Methods Med. Res. 2(1), 23–41 (1993)
C. Castillo-Chavez, B. Song, Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1(2), 361 (2004)
C. Briat, Sign properties of Metzler matrices with applications. Linear Algebra Appl. 515, 53–86 (2017)
T. Kaczorek, Positive stable realizations with system Metzler matrices, in Archives of Control Sciences (2011)
M. Ozair, A.A. Lashari, I.H. Jung, K.O. Okosun et al., Stability analysis and optimal control of a vector-borne disease with nonlinear incidence. Discrete Dyn. Nat. Soc. 2012 (2012)
G. Zaman, Y.H. Kang, I.H. Jung, Stability analysis and optimal vaccination of an sir epidemic model. Biosystems 93(3), 240–249 (2008)
B. Gomero, Latin hypercube sampling and partial rank correlation coefficient analysis applied to an optimal control problem (2012)
S. Marino, I.B. Hogue, C.J. Ray, D.E. Kirschner, A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254(1), 178–196 (2008)
Z. Neufeld, H. Khataee, A. Czirok, Targeted adaptive isolation strategy for covid-19 pandemic. Infect. Dis. Model. 5, 357–361 (2020)
G. Lahodny Jr., R. Gautam, R. Ivanek, Estimating the probability of an extinction or major outbreak for an environmentally transmitted infectious disease. J. Biol. Dyn. 9(sup1), 128–155 (2015)
G.E. Lahodny, L.J. Allen, Probability of a disease outbreak in stochastic multipatch epidemic models. Bull. Math. Biol. 75(7), 1157–1180 (2013)
P. Van den Driessche, J. Watmough, Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180(1–2), 29–48 (2002)
E. Michael, M.N. Malecela-Lazaro, J.W. Kazura, Epidemiological modelling for monitoring and evaluation of lymphatic filariasis control. Adv. Parasitol. 65, 191–237 (2007)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests upon publication of this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stephano, M.A., Irunde, J.I., Mayengo, M.M. et al. The randomness and uncertainty in dynamics of lymphatic filariasis: CTMC stochastic approach. Eur. Phys. J. Plus 139, 162 (2024). https://doi.org/10.1140/epjp/s13360-024-04945-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-024-04945-2