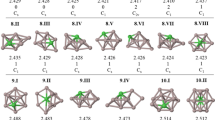
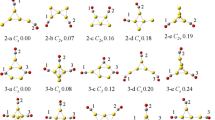
Abstract
Results of density functional theory calculations on Aln− and Aln−1Pt−, n = 2–8, clusters are presented and analyzed. The analysis includes different structural forms of the clusters characterized in terms of binding energy, spin and symmetry, and a comparative evaluation of various properties of the two systems viewed as connected through a single-Pt substitutional doping and examined in terms of their respective most stable structures. The Aln−1Pt− clusters are then used as a paradigmatic (model) case of single-atom nanocatalysts, with Pt as the catalytic center and Aln−1 as its support, to implement a uniform descriptor for gauging the tuning effects of all the parameters (“knobs”) of a nanocatalyst that include the identity of the active center and the material and size of its support.
Graphical abstract
















Similar content being viewed by others
Data Availability Statement
This manuscript has data included as electronic supplementary material. The online version of this article contains supplementary material, which is available to authorized users.
References
Nanoalloys: From Theory to Applications. Faraday Discussions No 138 (RCS Publishing, Cambridge, 2008).
R. Ferrando, J. Jellinek, R.L. Johnston, Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem. Rev. 108, 845–910 (2008)
M.M. Mariscal, O.A. Oviedo, E.P. Leiva (eds.), Metal Clusters and Nanoalloys—From Modeling to Applications (Springer, New York, 2016)
F. Calvo (ed.), Nanoalloys—From Fundamentals to Emergent Applications, 2nd edn. (Elsevier, Amsterdam, 2020)
P.H. Acioli, J. Jellinek, Theoretical analysis of photoelectron spectra of pure and mixed metal clusters: disentangling size, structure and composition effects. J. Phys. Chem. C 121, 16665–16672 (2017)
J. Jellinek, Nanoalloys: tuning properties and characteristics through size and composition. Faraday Discuss. 138, 11–35 (2008)
P.H. Acioli, X. Zhang, K.H. Bowen Jr., J. Jellinek, Electron binding energy spectra of AlnPt– clusters—a combined experimental and computational study. J. Phys. Chem. A 126, 4241–4247 (2022)
A.D. Becke, Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A At. Mol. Opt. Phys. 38, 3098–3100 (1988)
J.P. Perdew, Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B. 33, 8822–8824 (1986)
N. Godbout, D.R. Salahub, J. Andzelm, E. Wimmer, Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 70, 560–571 (1992)
C. Sosa, J. Andzelm, B.C. Elkin, E. Wimmer, K.D. Dobbs, D.A. Dixon, A local density functional study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 96, 6630–6636 (1992)
D. Andrae, U. Haeussermann, M. Dolg, H. Stoll, H. Preuss, Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chem. Acc. 77, 123–141 (1990)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery Jr., T. Vreven, K.N. Kudin, J.C. Burant et al., Gaussian 03, Revision C.02 (Gaussian Inc., Wallingford, 2004)
J. Jellinek, E.B. Krissinel, NinAlm alloy clusters: analysis of structural forms and their energy ordering. Chem. Phys. Lett. 258, 283–292 (1996)
K.J. Taylor, C.L. Pettiette, M.J. Craycraft, O. Chesnovsky, R.E. Smalley, Ups of negative aluminum clusters. Chem. Phys. Lett. 152, 347–352 (1988)
G. Ganteför, M. Gausa, K.-H. Meiwes-Broer, H.O. Lutz, Ultraviolet photodetachment spectroscopy on jet-cooled metal-cluster anions. Faraday Discuss. Chem. Soc. 86, 197–208 (1988)
U. Ray, M.F. Jarrold, J.E. Bower, J.S. Kraus, Photodissociation kinetics of aluminum cluster ions: determination of cluster dissociation energies. J. Chem. Phys. 91, 2912–2921 (1989)
C.-Y. Cha, G. Ganteför, W. Eberhardt, The development of the 3p and 4p valence band of small aluminum and gallium clusters. J. Chem. Phys. 100, 985–1010 (1994)
X. Li, H. Wu, X.-B. Wang, L.-S. Wang, s-p hybridization and electron shell structures in aluminum clusters: a photoelectron spectroscopy study. Phys. Rev. Lett. 81, 1909–1912 (1998)
J. Akola, M. Manninen, H. Häkkinen, U. Landman, X. Li, L.-S. Wang, Photoelectron spectra of aluminum cluster anions: temperature effects and ab initio simulations. Phys. Rev. B 60, R11297–R11300 (1999)
G.D. Geske, A.I. Boldyrev, X. Li, L.-S. Wang, On the origin of planarity in Al5− and Al5 clusters: the importance of a four-center peripheral bond. J. Chem. Phys. 113, 5130–5133 (2000)
J. Akola, M. Manninen, H. Häkkinen, U. Landman, X. Li, L.-S. Wang, Aluminum cluster anions: photoelectron spectroscopy and ab initio simulations. Phys. Rev. B 62, 13216–13228 (2000)
L. Ma, B. von Issendorff, A. Aguado, Photoelectron spectroscopy of cold aluminum cluster anions: comparison with density functional theory results. J. Chem. Phys. 132, 104303 (2010)
J.J. Melko, A.W. Castleman Jr., Photoelectron imaging of small aluminum clusters: quantifying s–p hybridization. Phys. Chem. Chem. Phys. 15, 3173–3178 (2013)
B. Kafle, V. Chandrasekaran, O. Heber, M.L. Rappaport, H. Rubenstein, D. Schwalm, D. Strasser, D. Zajfman, Electron detachment and fragmentation of laser-excited rotationally hot Al4−. Phys. Rev. A 92, 052503 (2015)
A. Rubio, L.C. Balbas, J.A. Alonso, Electronic structure of negatively charged aluminium clusters. Phys. B 1991(168), 32–38 (1991)
B.K. Rao, P. Jena, Evolution of the electronic structure and properties of neutral and charged aluminum clusters: a comprehensive analysis. J. Chem. Phys. 111, 1890–1904 (1999)
O. Dolgounitcheva, V.G. Zakrzewski, J.V. Ortiz, Ground state and vertical electron detachment energies of icosahedral and D5h Al13−. J. Chem. Phys. 111, 10762–10765 (1999)
N. Drebov, R. Ahlrichs, Structures of Aln, its anions and cations up to n=34: a theoretical investigation. J. Chem. Phys. 132, 164703 (2010)
L. Candido, J.N. Teixeira Rabelo, J.L.F. Da Silva, G.-Q. Hai, Quantum Monte Carlo study of small aluminum clusters Aln(n=2–13). Phys. Rev. B 85, 245404 (2012)
S. Paranthaman, K. Hong, J. Kim, D.E. Kim, T.K. Kim, Density functional theory assessment of molecular structures and energies of neutral and anionic Aln (n=2-10) clusters. J. Phys. Chem. A 2013(117), 9293–9303 (2013)
L.-P. Tan, D. Die, B.-X. Zheng, Growth mechanism, electronic properties and spectra of aluminum clusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 267, 120545 (2022)
X. Zhang, G. Ganteför, K.H. Bowen Jr., A.N. Alexandrova, The PtAl− and PtAl2− anions: theoretical and photoelectron spectroscopic characterization. J. Chem. Phys. 140, 164316 (2014)
W.P. Wijesundera, Theoretical study of the negative ions of boron, aluminum, gallium, indium, and thallium. Phys. Rev. A 55, 1785–1791 (1997)
S.S. Ray, R.K. Chaudhuri, S. Chattopadhyay, Communication: viewing the ground and excited electronic structures of platinum and its ion through the window of relativistic coupled cluster method. J. Chem. Phys. 146, 011102 (2017)
J.C. Rienstra-Kiracofe, G.S. Tschumper, H.F. Schaefer III., S. Nandi, B. Ellison, Atomic and molecular electron affinities: photoelectron experiments and theoretical computations. Chem. Rev. 102, 231–282 (2002)
M. Scheer, R.C. Bilodeau, J. Thogersen, H.K. Haugen, Threshold photodetachment of Al−: electron affinity and fine structure. Phys. Rev. A 57, R1493–R1496 (1998)
C.E. Moore, Atomic Energy Levels: As Derived from the Analyses of Optical Spectra, Vol II 24Cr-41Nb (Natl. Bur. Stand. Circ. 467, US GPO, Washington, D.C., 1949, 1952)
H. Hotop, W.C. Lineberger, Binding energies in atomic negative ions: II. J. Phys. Chem. Ref. Data 1985(14), 731–750 (1985)
W. Kohn, L.J. Sham, Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965)
J. Jellinek, P.H. Acioli, Converting Kohn–Sham eigenenergies into electron binding energies. J. Chem. Phys. 118, 7783 (2003)
A.E. Reed, F. Weinhold, natural bond orbital analysis of near-Hartree–Fock water dimer. J. Chem. Phys. 78, 4066–4073 (1983)
A.E. Reed, R.B. Weinstock, F. Weinhold, Natural population analysis. ibid. 83, 735–746 (1985)
X.-F. Yang, A. Wang, B. Qiao, J. Li, J. Liu, T. Zhang, Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013)
B. Hammer, J.K. Norskov, Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 343, 211–220 (1995)
B. Hammer, J.K. Norskov, Theoretical surface science and catalysis—calculations and concepts. Adv. Cat. 45, 71–129 (2000)
Acknowledgements
The work at Argonne was supported by the Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences, U.S. Department of Energy under Contract No. DE-AC02-06CH11357 (J.J.) This research used in part the resources of the National Energy Research Scientific Computing Center (NERSC) supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to the paper.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Acioli, P.H., Jellinek, J. A computational study of Aln− and Aln−1Pt− clusters: the effects of doping and a uniform tuning gauge for single-atom nanocatalysts. Eur. Phys. J. D 76, 230 (2022). https://doi.org/10.1140/epjd/s10053-022-00556-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjd/s10053-022-00556-7