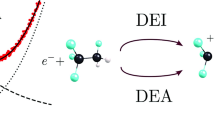
Abstract
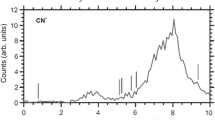
We experimentally probed the dissociation of methyl isocyanide, CH\(_3\)NC, via low-energy electron attachment. We have measured the absolute dissociative electron attachment (DEA) cross section for each of the produced ions as a function of incident electron energy. We were able to observe the CH\(_2^-\), CH\(_3^-\), CN\(^-\), CNC\(^-\), CHNC\(^-\) and CH\(_2\)NC\(^-\) anionic fragments as DEA products. Our experimental results are consistent with a previous report, and in addition, we observed the CH\(_2^-\) anion for the first time. To support these experimental results, we also have performed density functional theory (DFT) calculation at the B3LYP/aug-cc-pVTZ level of theory to find out which reaction channel(s) lead to the formation of a given anion.
Graphic Abstract



Similar content being viewed by others
Data Availability Statement
This manuscript has no associated data or the data will not be deposited. [Authors’ comment: There are no associated data available.]
References
R.A. Alvarez, C.B. Moore, Science 263, 205 (1994). https://science.sciencemag.org/content/263/5144/205.full.pdf
S.G. Antonsen, A.J.C. Bunkan, T. Mikoviny, C.J. Nielsen, Y. Stenstrøm, A. Wisthaler, E. Zardin, J. Phys. Chem. A 124, 6562 (2020). https://doi.org/10.1021/acs.jpca.0c05127 (pMID: 32663395)
W.M. Irvine, F.P. Schloerb, Astrophys. J. 282, 516 (1984)
A.J. Remijan, J.M. Hollis, F.J. Lovas, D.F. Plusquellic, P.R. Jewell, Astrophys. J. 632, 333 (2005)
H. Calcutt, M.R. Fiechter, E.R. Willis, H.S.P. Müller, R.T. Garrod, J.K. Jørgensen, S.F. Wampfler, T.L. Bourke, A. Coutens, M.N. Drozdovskaya et al., A&A 617, A95 (2018)
A. Mariani, D.A. Russell, T. Javelle, J.D. Sutherland, J. Am. Chem. Soc. 140, 8657 (2018). https://doi.org/10.1021/jacs.8b05189 (pMID: 29965757)
M. Heni, E. Illenberger, Int. J. Mass Spectrom. Ion Process. 73, 127 (1986)
W. Sailer, A. Pelc, P. Limão-Vieira, N. Mason, J. Limtrakul, P. Scheier, M. Probst, T. Märk, Chem. Phys. Lett. 381, 216 (2003)
J.A. Stockdale, F.J. Davis, R.N. Compton, C.E. Klots, J. Chem. Phys. 60, 4279 (1974). https://doi.org/10.1063/1.1680900
H. Li, X.F. Gao, X. Meng, S.X. Tian, J. Phys. Chem. A 123, 9089 (2019). https://doi.org/10.1021/acs.jpca.9b07399 (pMID: 31525926)
T.F.M. Luxford, J. Kočišek, L. Tiefenthaler, P. Nag, Eur. Phys. J. D 75, 230 (2021)
A.P. Hitchcock, M. Tronc, A. Modelli, J. Phys. Chem. 93, 3068 (1989). https://doi.org/10.1021/j100345a039
F. Edard, A.P. Hitchcock, M. Tronc, J. Phys. Chem. 94, 2768 (1990). https://doi.org/10.1021/j100370a010
M. Gochel-Dupuis, J. Delwiche, M.J. Hubin-Franskin, J.E. Collin, F. Edard, M. Tronc, J. Am. Chem. Soc. 112, 5425 (1990). https://doi.org/10.1021/ja00170a005
R. Janečková, D. Kubala, O. May, J. Fedor, M. Allan, Phys. Rev. Lett. 111, 213201 (2013)
M. Zawadzki, T.F.M. Luxford, J. Kočišek, J. Phys. Chem. A 124, 9427 (2020). https://doi.org/10.1021/acs.jpca.0c07283 (pMID: 33125242)
P. Nag, M. Polášek, J. Fedor, Phys. Rev. A 99, 052705 (2019)
P. Nag, M. Tarana, J. Fedor, Phys. Rev. A 103, 032830 (2021)
E. Krishnakumar, S.K. Srivastava, J. Phys. B: Atomic Molecular Optical Phys. 21, 1055 (1988)
M. Stepanović, Y. Pariat, M. Allan, J. Chem. Phys. 110, 11376 (1999). https://doi.org/10.1063/1.479078
J. Langer, M. Zawadzki, M. Fárník, J. Pinkas, J. Fedor, J. Kočišek, Eur. Phys. J. D 72, 112 (2018)
M. Ranković, P. Nag, M. Zawadzki, L. Ballauf, J. Žabka, M. Polášek, J. Kočišek, J. Fedor, Phys. Rev. A 98, 052708 (2018)
J. Fedor, O. May, M. Allan, Phys. Rev. A 78, 032701 (2008)
M. Allan, J. Electron Spectrosc. Related Phenom. 48, 219 (1989)
R. Dressler, M. Allan, Chem. Phys. 92, 449 (1985)
P. Nag, D. Nandi, Phys. Rev. A 91, 052705 (2015)
O. Orient, S. Srivastava, Chem. Phys. Lett. 96, 681 (1983)
D. Rapp, D.D. Briglia, J. Chem. Phys. 43, 1480 (1965). https://doi.org/10.1063/1.1696958
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, G.A. Petersson, H. Nakatsuji et al., Gaussian\({\tilde{\,}}^{}16\) Revision C.01 (2016), gaussian Inc. Wallingford CT
R. Janečková, O. May, J. Fedor, Phys. Rev. A 86, 052702 (2012)
A.D. Bass, J.H. Bredehöft, E. Böhler, L. Sanche, P. Swiderek, Eur. Phys. J. D 66, 53 (2012)
E. Illenberger, J. Momigny, Gaseous Molecular Ions: An Introduction to Elementary Processes Induced by Ionization (Steinkopff, Heidelberg, 1992)
Acknowledgements
The authors acknowledge Dr Juraj Fedor and Dr Jaroslav Koĉiŝek for valuable suggestions and fruitful discussion throughout the entire stage of the present work. This work has been supported by the Czech Science Foundation Project Nr. 20-11460S and Ministry of Education, Youth and Sport of the Czech Republic Project Nr. LTAUSA19031.
Author information
Authors and Affiliations
Contributions
TFML performed the experiments with TEM-QMS set-up and the quantum chemical calculations. PN performed the experiments with the DEA-VMI set-up. Both the authors prepared the manuscript and approved it.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luxford, T.F.M., Nag, P. Dissociative electron attachment to methyl isocyanide. Eur. Phys. J. D 75, 270 (2021). https://doi.org/10.1140/epjd/s10053-021-00278-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjd/s10053-021-00278-2