Abstract
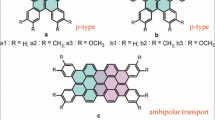
In this work, the charge transport properties of Isoindigo (II) and its derivatives which have the same hexyl chain were theoretically investigated by the Marcus-Hush theory combined with density functional theory (DFT). Here we demonstrate that the changes of benzene and thiophene groups in molecular structure have an important influence on the charge transport properties of organic semiconductor. The benzene rings of II are replaced by thiophenes to form the thienoisoindigo (TII), and the addition of benzene rings to the TII form the benzothienoisoindigo (BTII). The results show that benzene rings and thiophenes change the chemical structure of crystal molecules, which lead to different molecule stacking, thus, the length of hydrogen bond was changed. A shorter intermolecular hydrogen bond lead to tighter molecular stacking, which reduces the center-to-center distance and enhances the ability of charge transfer. At the same time, we theoretically demonstrated that II and BTII are the ambipolar organic semiconductor. BTII has better carrier mobility. The hole mobility far greater than electron mobility in TII, which is p-type organic semiconductor. Among all hopping path, we find that the distance of face-to-face stacking in II is the shortest and the electron-transport electronic coupling V e is the largest, but II has not a largest anisotropic mobility, because the reorganization energy has a greater influence on the mobility than the electronic coupling. This work is helpful for designing ambipolar organic semiconductor materials with higher charge transport properties by introducing benzene ring and thiophene.
Graphical abstract

Similar content being viewed by others
References
X. Chi, D. Li, H. Zhang, Y. Chen, V. Garcia, C. Garcia, T. Siegrist, Org. Electron. 9, 234 (2008)
G.H. Gelinck, T.C.T. Geuns, D.M. De Leeuw, Appl. Phys. Lett. 77, 1487 (2000)
M. Muccini, Nat. Mater. 5, 605 (2006)
S. Nagamatsu, K. Kaneto, R. Azumi, M. Matsumoto, Y. Yoshida, K. Yase, J. Phys. Chem. B 109, 9374 (2005)
N. Padma, S.N. Sawant, S. Sen, Mater. Sci. Semicond. Proc. 30, 18 (2015)
G. Barbarella, M. Melucci, G. Sotgiu, Adv. Mater. 17, 1581 (2005)
A.C. Grimsdale, K.L. Chan, R.E. Martin, P.G. Jokisz, A.B. Holmes, Chem. Rev. 109, 897 (2009)
Y. Shirota, H. Kageya, Chem. Rev. 107, 953 (2007)
Y.J. Cheng, S.H. Yang, C.S. Hsu, Chem. Rev. 109, 5868 (2009)
S. Günes, H. Neugebauer, N.S. Sariciftci, Cheminform 38, 1324 (2007)
G. Ren, P.T. Wu, S.A. Jenekhe, Chem. Mater. 22, 2020 (2010)
S. Fabiano, H. Usta, R. Forchheimer, X. Crispin, A. Facchetti, M. Berggren, Adv. Mater. 26, 7438 (2015)
T.D. Anthopoulos, G.C. Anyfantis, G.C. Papavassiliou, D.M.D. Leeuw, Appl. Phys. Lett. 90, 678 (2007)
E.D. Głowacki, M. Irimiavladu, M. Kaltenbrunner, J. Gsiorowski, M.S. White, U. Monkowius, G. Romanazzi, G.P. Suranna, P. Mastrorilli, T. Sekitani, Adv. Mater. 25, 1563 (2013)
M. Irimia-Vladu, P.A. Troshin, M. Reisinger, L. Shmygleva, Y. Kanbur, G. Schwabegger, M. Bodea, R. Schwödiauer, A. Mumyatov, J.W. Fergus, Adv. Funct. Mater. 20, 4017 (2010)
E.D. Głowacki, D.H. Apaydin, Z. Bozkurt, U. Monkowius, K. Demirak, E. Tordin, M. Himmelsbach, C. Schwarzinger, M. Burian, R.T. Lechner, J. Mater. Chem. C 2, 8089 (2014)
T. Hasegawa, M. Ashizawa, H. Matsumoto, RSC Adv. 5, 61035 (2015)
L. Liu, G. Yang, Y. Duan, Y. Geng, Y. Wu, Z. Su, Org. Electron. 15, 1896 (2014)
A.J. Alexander, R.N. Zare, Acc. Chem. Res. 33, 199 (2000)
Y.D. Wu, W. Han, D.P. Wang, Y. Gao, Y.L. Zhao, Acc. Chem. Res. 41, 1418 (2008)
G.J. Zhao, K.L. Han, J. Phys. Chem. A 113, 14329 (2009)
G.J. Zhao, J.Y. Liu, A. Lichuan Zhou, K.L. Han, J. Phys. Chem. B 111, 8940 (2007)
H.L. Wei, Y.R. Shi, Y.F. Liu, Semicond. Sci. Technol. 31, 065016 (2016)
R. Marcus, J. Chem. Phys. 26, 872 (1963)
N.S. Hush, J. Chem. Phys. 28, 962 (1958)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Ö. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09, Revision A.02 (Gaussian, Inc., Wallingford, CT, 2009)
J.J. Kwiatkowski, J. Nelson, H. Li, J.L. Bredas, W. Wenzel, C. Lennartz, Phys. Chem. Chem. Phys. 10, 1852 (2008)
T. Yamada, T. Sato, K. Tanaka, H. Kaji, Org. Electron. 11, 255 (2010)
G.R. Hutchison, M.A. Ratner, T.J. Marks, J. Am. Chem. Soc. 127, 2339 (2005)
V. Coropceanu, T. Nakano, N.E. Gruhn, O. Kwon, T. Yade, K. Katsukawa, J.L. Brédas, J. Phys. Chem. B 110, 9482 (2006)
J.L. Bredas, D. Beljonne, V. Coropceanu, J. Cornil, Chem. Rev. 104, 4971 (2004)
G. Te Velde, F.M. Bickelhaupt, E.J. Baerends, C. Fonseca Guerra, S.J.A. Van Gisbergen, J.G. Snijders, T. Ziegler, J. Comput. Chem. 22, 931 (2001)
K. Senthilkumar, F.C. Grozema, F.M. Bickelhaupt, L.D.A. Siebbeles, J. Chem. Phys. 119, 9809 (2003)
V.C. Sundar, J. Zaumseil, V. Podzorov, E. Menard, R.L. Willett, T. Someya, M.E. Gershenson, J.A. Rogers, Science 303, 1644 (2004)
S.H. Wen, A. Li, J. Song, W.Q. Deng, K.L. Han, W.A.G. Iii, J. Phys. Chem. B 113, 8813 (2009)
K. Ming-Yu, H.Y. Chen, I. Chao, Chemistry 13, 4750 (2007)
S. Mohakud, A.P. Alex, S.K. Pati, J. Phys. Chem. C 114, 20436 (2010)
A. Chandekar, J.E. Whitten, Synth. Met. 150, 259 (2005)
X.Q. Ran, J.K. Feng, Y.L. Liu, A.M. Ren, L.Y. Zou, C.C. Sun, J. Phys. Chem. A 112, 10904 (2008)
X.Y. Zhang, G.J. Zhao, J. Phys. Chem. C 116, 13858 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, XB., Wei, HL., Shi, YT. et al. Theoretical studies on the effect of benzene and thiophene groups on the charge transport properties of Isoindigo and its derivatives. Eur. Phys. J. D 71, 314 (2017). https://doi.org/10.1140/epjd/e2017-80211-x
Received:
Revised:
Published:
DOI: https://doi.org/10.1140/epjd/e2017-80211-x