Abstract.
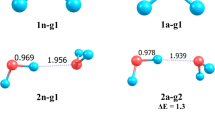
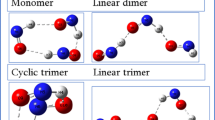
Electron localization is studied in formamide cluster anions. The isolated formamide molecule has a large dipole moment and its clusters can give birth to multipole-bound anions as well as valence anions. The vertical valence electron affinity of the isolated molecule is determined by electron transmission spectroscopy. The anion formation process is studied as a function of cluster size with Rydberg electron transfer spectroscopy. DFT calculations of the neutral and negatively-charged cluster structures show that the anion excess electron localizes on a single molecule. The adiabatic valence electron affinity of isolated formamide is deduced from the observation of the cluster size threshold for valence attachment.
Similar content being viewed by others
References
E. Fermi, E. Teller, Phys. Rev. 72, 399 (1947)
P. Skurski, J. Simons, J. Chem. Phys. 112, 6562 (2000)
H. Haberland, H. Ludewigt, C. Schindler, D.R. Worsnop, Phys. Rev. A 36, 967 (1987)
J. Kalcher, Theoretical prospects of negative ions (Research SignPost, Trivandrum, India, 2002)
J.R.R. Verlet, A.E. Bragg, A. Kammrath, O. Chesnowsky, D.M. Neumark, Science 307, 93 (2005)
E. Illenberger, Chem. Rev. 92, 1589 (1992)
H. Abdoul-Carime, J.P. Schermann, C. Desfrançois, Few-Body Systems 31, 183 (2002)
H.M. Lee, S.B. Suh, K.S. Kim, J. Chem. Phys. 118, 9981 (2003)
J.W. Shin, N.I. Hammer, J.M. Headrick, M.A. Johnson, Chem. Phys. Lett. 399, 349 (2004)
H. Haberland, C. Schindler, H.G. Worsnop, Ber. Bunsenges. Phys. Chem. 88, 270 (1984)
J.V. Coe, G.H. Lee, J.G. Eaton, S.T. Arnold, H.W. Sarkas, K.H. Bowen, C. Ludewigt, H. Haberland, D.R. Worsnop, J. Chem. Phys. 92, 3980 (1990)
C. Desfrançois, A. Lisfi, J.P. Schermann, Z. Phys. D 24, 297 (1992)
G.J. Schulz, Rev. Mod. Phys. 45, 378 (1973)
L. Sanche, G.J. Schulz, Phys. Rev. A 5, 1672 (1972)
K. Harth, M.-W. Rüf, H. Hotop, Z. Phys. D 14, 149 (1989)
C. Desfrançois, V. Périquet, S. Carles, J.P. Schermann, L. Adamowicz, Chem. Phys. 239, 475 (1998)
B. Lucas, F. Lecomte, B. Reimann, H.D. Barth, G. Grégoire, Y. Bouteiller, J.P. Schermann, C. Desfrançois, Phys. Chem. Chem. Phys. 6, 2600 (2004)
C.C. Wu, J.C. Jiang, I. Hahndorf, C. Chaudhuri, Y.T. Lee, H.C. Chang, J. Phys. Chem. A 104, 9556 (2000)
T. Maeyama, N. Mikami, Phys. Chem. Chem. Phys. 6, 1137 (2003)
A. Modelli, D. Jones, G. Distefano, Chem. Phys. Lett. 86, 434 (1982)
A.R. Johnston, P.D. Burrow, J. Electron. Spectrosc. Relat. Phenom. 25, 119 (1982)
P.D. Burrow, J.A. Michejda, Chem. phys. Lett. 42, 223 (1976)
A. Modelli, G. Distefano, D. Jones, Chem. Phys. 73, 395 (1982)
A. Modelli, H.D. Martin, J. Phys. Chem. A 106, 7271 (2002)
F.B. Dunning, J. Phys. B 28, 1645 (1995)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, H.B. Scuseria, G.E. Robb, M.A. Cheeseman, J.R. Zakrzewski, V.G. Montgomery, J.A. Stratmann, R.E. Burant, J.C. Dapprich, S. Millam, J.M. Daniels, A.D. Kudin, K.N. Strain, M.O. Farkas, O. Tomasi, J. Barone, V. Cossi, M. Cammi, R. Mennucci, B. Pomelli, C. Adamo, C. Clifford, S. Ochterski, J. Petersson, G.A. Ayala, Q.P.Y. Cui, K. Morokuma, K. Malick, D.K. Rabuck, A.D. Raghavachari, K. Foresman, J.B. Cioslowski, J. Ortiz, J.V. Stefanov, B.B. Liu, G. Liashenko, A. Piskorz, P. Komaromi, I. Gomperts, R. Martin, R.L. Fox, D.J. Keith, T. Al-Laham, M.A. Peng, C.Y. Nanayakkara, A. Gonzalez, C. Challacombe, M. Gill, P.M.W. Johnson, B. Chen, W. Wong, M.W. Andres, J.L. Head-Gordon, E.S. Replogle, J.A. Popple, Revision A6 ed. (Pittsburg, PA, 1998)
C. Desfrançois, Y. Bouteiller, J.P. Schermann, D. Radisic, S.T. Stockes, K.H. Bowen, N.I. Hammer, R.N. Compton, Phys. Rev. Lett. 92, 083003 (2004)
A. Modelli, Trends Chem. Phys. 6, 57 (1997)
D. Chen, G.A. Gallup, J. Chem. Phys. 93, 8893 (1990)
S.S. Staley, J.T. Strnad, J. Phys. Chem. 98, 161 (1994)
J.S. Chao, M.F. Falcetta, K.D. Jordan, J. Chem. Phys. 93, 1125 (1990)
A. Modelli, Phys. Chem. Chem. Phys. 5, 2923 (2003)
A. Modelli, B. Hajgato, J.F. Nixon, L. Nyulaszi, J. Phys. Chem. A 108, 7440 (2004)
N. Heinrich, W. Koch, G. Frenking, Chem. Phys. Lett. 124, 20 (1986)
A. Pelc, W. Sailer, P. Scheier, M. Probst, N.J. Mason, E. Illenberger, T.D. Märk, Chem. Phys. Lett. 361, 277 (2002)
J.W. Shin, N.I. Hammer, M.A. Johnson, H. Schneider, A. Glob, J.M. Weber, J. Phys. Chem. A (2005)
V. Périquet, A. Moreau, S. Carles, J.P. Schermann, C. Desfrançois, J. Electr. Spectr. Rel. Phenom. 106, 141 (2000)
K.T. No, O.Y. Kwon, S.Y. Kim, M.S. Jhon, H.A. Scheraga, J. Phys. Chem. 99, 3478 (1995)
R. Ludwig, F. Weinhold, T.C. Farrar, J. Chem. Phys. 103, 3636 (1995)
M.C. Bellissent-Funel, S. Nasr, L. Bosio, J. Chem. Phys. 106, 7913 (1997)
C.N. Tam, P. Bour, J. Eckert, F.R. Trouw, J. Phys. Chem. A 101, 5877 (1997)
K.P. Sagarik, R. Ahlrichs, J. Phys. Chem. 86, 5117 (1987)
S. Suhai, J. Chem. Phys. 103, 7030 (1995)
S. Suhai, J. Phys. Chem. 100, 3950 (1996)
E. Cabaleiro-Lago, M.A. Rios, J. Chem. Phys. 110, 6782 (1999)
J.B. Foresman, A. Frisch, Exploring Chemistry with Electronic Structure methods (Gauusian, Inc, Pittsburg, US, 1996)
C. Desfrançois, H. Abdoul-Carime, J.P. Schermann, Int. J. Mod. Phys. 10, 1339 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seydou, M., Modelli, A., Lucas, B. et al. Electron attachment to strongly polar clusters. Eur. Phys. J. D 35, 199–205 (2005). https://doi.org/10.1140/epjd/e2005-00089-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjd/e2005-00089-5