Abstract:
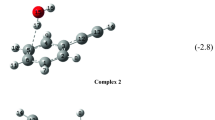
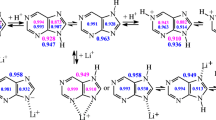
An experimental and theoretical RHF, MP2 and DFT/6-31++G** study is described of the matrix FT-IR spectra of monomer 2-aminopurine and H-bonded complexes of 2-aminopurine with water. 2-aminopurine occurs in Ar predominantly as the amino-N9H tautomer, but small amounts of the amino-N7H tautomer are also present. An approximate KT value for this tautomeric equilibrium is found to be 0.016 (RHF) and 0.015 (DFT) using the infrared intensity measurement. Four H-bonded complexes of the abundant amino-N9H form with water are detected in the experimental FT-IR spectrum by their characteristic predicted absorptions, i.e. the three closed complexes N3 ... H-O ... H-N9, N1 ... H-O ... H-NH, N3 ... H-O ... H-NH and the open complex N7 ... H-OH. From the experimental results, the proton affinity of the N7 atom in 2-aminopurine can be estimated. The dependence of the H-bond strength on the H-bond linearity is demonstrated by a correlation between the N ... H distance and the N ... H-O angle in closed N ... H-O ... H-N complexes.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 10 December 2001 Published online 13 September 2002
Rights and permissions
About this article
Cite this article
Ramaekers, R., Adamowicz, L. & Maes, G. Tautomery and H-bonding characteristics of 2-aminopurine: a combined experimental and theoretical study. Eur. Phys. J. D 20, 375–388 (2002). https://doi.org/10.1140/epjd/e2002-00160-9
Issue Date:
DOI: https://doi.org/10.1140/epjd/e2002-00160-9