Abstract
The pollution of the atmospheric environment has significantly increased due to the rapid growth of population and industrial development. To prevent environmental disasters caused by such deterioration, it is imperative to control and monitor such pollutants. This study investigates the structural, electronic, and optical characteristics of the CdS monolayer nanosheets, and evaluates their gas adsorption behavior for various gas molecules (CO2, SO2, H2S, CO, and SO) using DFT calculations. SO2, H2S, and CO are found to show weak adsorption suggesting the physisorption behavior, whereas CO2 and SO adsorption systems are found to undergo chemical adsorption on the CdS surface, suggesting it as a promising sensing material for the latter gasses. The electronic structure and geometrical positions of the gas molecules on monolayer CdS surface have been analyzed, revealing a significant alteration in the band gap of CdS upon the the adsorption of gas molecules. We also investigated the optical properties of the monolayer CdS in the presence of the gasses, indicating that the real and imaginary components of the dielectric function are crucial for sensing applications, and changes in the absorption spectrum of the CdS monolayer upon adsorption of the different gasses can be attributed to the specific electronic and chemical properties of each gas and their interaction with the CdS surface.
Graphical Abstract

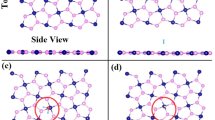
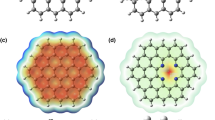
The analysis of the electron localization function (ELF) and charge density differences (CDD) is shown here for CO/CdS and SO/CdS systems. The high degree of ELF and CDD indicates chemisorption (SO/CdS system), while a low degree (or negligible) of ELF and CDD suggests physisorption (CO/CdS system)











Similar content being viewed by others
Data availability
This manuscript has no associated data or the data will not be deposited. [Authors' comment: The authors confirm that the data supporting the findings of this study are available within the article.]
References
M.V. Nikolic, V. Milovanovic, Z.Z. Vasiljevic, Z. Stamenkovic, Semiconductor gas sensors: materials, technology, design, and application. Sensors (Switzerland) 20, 1–31 (2020). https://doi.org/10.3390/s20226694
T. Alaa Hussein, N.M. Shiltagh, W. Kream Alaarage, R.R. Abbas, R.A. Jawad, A.H. Abo Nasria, Electronic and optical properties of the BN bilayer as gas sensor for CO2, SO2, and NO2 molecules: a DFT study. Results Chem 5, 100978 (2023). https://doi.org/10.1016/j.rechem.2023.100978
A. Abojassim, Z. Gateaa, A. Abo Nasria, Adsorption of gas molecules on (BN) monolayer as potential SO, SO2 NO, and NO2 gases sensor: a DFT study. J. Chem. 65, 1–11 (2021). https://doi.org/10.21608/ejchem.2021.92587.4380
W. Kream Alaarage, A.H. Abo Nasria, A.H. Omran Alkhayatt, A DFT investigation of an InP bilayer: a potential gas sensor with promising adsorption and optical response. Comput. Theor. Chem. 1227, 114223 (2023). https://doi.org/10.1016/j.comptc.2023.114223
P.T. Moseley, Solid state gas sensors. Meas. Sci. Technol. 8, 223–237 (1997). https://doi.org/10.1088/0957-0233/8/3/003
T.A. Hussein, W.K. Alaarage, H.A. Abdulhussein, N. Seriani, A.H. Abo Nasria, Ga-doped AlN monolayer nano-sheets as promising materials for environmental sensing applications. Comput. Theor. Chem. 1223, 114086 (2023). https://doi.org/10.1016/j.comptc.2023.114086
Y. Kang, F. Yu, L. Zhang, W. Wang, L. Chen, Y. Li, Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 360, 1155 (2021). https://doi.org/10.1016/j.ssi.2020.115544
S. Steinhauer, Gas sensors based on copper oxide nanomaterials: a review. Chemosensors. 9, 1–20 (2021). https://doi.org/10.3390/chemosensors9030051
Y. Masuda, Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B Chem. 364, 131876 (2022). https://doi.org/10.1016/j.snb.2022.131876
T.V. Perevalov, I.P. Prosvirin, E.A. Suprun, F. Mehmood, T. Mikolajick, U. Schroeder, V.A. Gritsenko, J. Sci. Adv. Mater. Devices films 6, 1–6 (2021)
S. Elouali, L.G. Bloor, R. Binions, I.P. Parkin, C.J. Carmalt, J.A. Darr, Gas sensing with nano-indium oxides (In2O3) prepared via continuous hydrothermal flow synthesis. Langmuir 28, 1879–1885 (2012). https://doi.org/10.1021/la203565h
Z. Wei, L. Xu, S. Peng, Q. Zhou, Application of WO3 hierarchical structures for the detection of dissolved gases in transformer oil: a mini review. Front. Chem. 8, 6–11 (2020). https://doi.org/10.3389/fchem.2020.00188
T. Lin, X. Lv, S. Li, Q. Wang, The morphologies of the semiconductor oxides and their gas-sensing properties. Sensors (Switzerland) 17, 1–30 (2017). https://doi.org/10.3390/s17122779
R. Woods-Robinson, Y. Han, H. Zhang, T. Ablekim, I. Khan, K.A. Persson, A. Zakutayev, Wide band gap chalcogenide semiconductors. Chem. Rev. 120, 4007–4055 (2020). https://doi.org/10.1021/acs.chemrev.9b00600
G. Volonakis, N. Sakai, H.J. Snaith, F. Giustino, Oxide analogs of halide perovskites and the new semiconductor Ba2AgIO6. J. Phys. Chem. Lett. 10, 1722–1728 (2019). https://doi.org/10.1021/acs.jpclett.9b00193
N.F. Mott, Silicon dioxide and the chalcogenide semiconductors; similarities and differences. Adv. Phys. 26, 363–391 (1977). https://doi.org/10.1080/00018737700101413
S.V. Dmitriev, I.V. Dementiev, Vitreous chalcogenide semiconductors for gas sensing application. MRS Proc. (2004). https://doi.org/10.1557/PROC-828-A5.8
M. Mitkova, D. Butt, M. Kozicki, H. Barnaby, Chalcogenide Glass Radiation Sensor; Materials Development, Design and Device Testing (Idaho Falls, ID, USA, 2013)
J.S. Jie, W.J. Zhang, Y. Jiang, X.M. Meng, Y.Q. Li, S.T. Lee, Photoconductive characteristics of single-crystal CdS nanoribbons. Nano Lett. 6, 1887–1892 (2006). https://doi.org/10.1021/nl060867g
S.T. Navale, A.T. Mane, M.A. Chougule, N.M. Shinde, J. Kim, V.B. Patil, Highly selective and sensitive CdS thin film sensors for detection of NO2 gas. RSC Adv. 4, 44547–44554 (2014). https://doi.org/10.1039/c4ra06531j
H. Xia, J. Peng, K. Liu, C. Li, Y. Fang, Preparation and gas sensing properties of novel CdS-supramolecular organogel hybrid films. J. Phys. D Appl. Phys. 41, 105405 (2008). https://doi.org/10.1088/0022-3727/41/10/105405
L. Yadava, R. Verma, R. Dwivedi, Sensing properties of CdS-doped tin oxide thick film gas sensor. Sens. Actuators B Chem. 144, 37–42 (2010). https://doi.org/10.1016/j.snb.2009.10.013
D.S. Dhawale, D.P. Dubal, V.S. Jamadade, R.R. Salunkhe, S.S. Joshi, C.D. Lokhande, Room temperature LPG sensor based on n-CdS/p-polyaniline heterojunction. Sens. Actuators B Chem. 145, 205–210 (2010). https://doi.org/10.1016/j.snb.2009.11.063
X. Fu, J. Liu, Y. Wan, X. Zhang, F. Meng, J. Liu, Preparation of a leaf-like CdS micro-/nanostructure and its enhanced gas-sensing properties for detecting volatile organic compounds. J. Mater. Chem. 22, 17782–17791 (2012). https://doi.org/10.1039/c2jm33352j
B.T. Raut, P.R. Godse, S.G. Pawar, M.A. Chougule, V.B. Patil, Development of nanostructured CdS sensor for H2S recognition: structural and physical characterization. J. Mater. Sci. Mater. Electron. 23, 956–963 (2012). https://doi.org/10.1007/s10854-011-0527-2
T. Arai, T. Yoshida, T. Ogawa, Photoacoustic and luminescence spectra of CdS fine particles. Jpn. J. Appl. Phys. 26, 396–404 (1987). https://doi.org/10.1143/JJAP.26.396
B. Su, K.L. Choy, Microstructure and properties of the CdS thin films prepared by electrostatic spray assisted vapour deposition (ESAVD) method. Thin Solid Films 359, 160–164 (2000). https://doi.org/10.1016/S0040-6090(99)00733-6
H.H. Afify, I.K. Battisha, Oxygen interaction with CdS based gas sensors by varying different preparation parameters. J. Mater. Sci. Mater. Electron. 1, 373–377 (2000)
R. Demir, S. Okur, M. Şeker, Electrical characterization of CdS nanoparticles for humidity sensing applications. Ind. Eng. Chem. Res. 51, 3309–3313 (2012). https://doi.org/10.1021/ie201509a
Y. Cao, L. Guo, M. Dan, D.E. Doronkin, C. Han, Z. Rao, Y. Liu, J. Meng, Z. Huang, K. Zheng, P. Chen, F. Dong, Y. Zhou, Modulating electron density of vacancy site by single Au atom for effective CO2 photoreduction. Nat. Commun. (2021). https://doi.org/10.1038/s41467-021-21925-7
Y. Li, D. Bahamon, M. Sinnokrot, L.F. Vega, Insights into the mechanisms of H2S adsorption and dissociation on CdS surfaces by DFT-D3 calculations. Int. J. Hydrogen Energy 48(26), 9700–9712 (2022)
Y. Li, Computational screening of transition metal-doped CdS for photocatalytic hydrogen production. npj Comput. Mater. (2022). https://doi.org/10.1038/s41524-022-00922-4
M. Li, H. Zhu, G. Wei, A. He, Y. Liu, DFT calculation and analysis of the gas sensing mechanism of methoxy propanol on Ag decorated SnO2 (110) surface. RSC Adv. 9(61), 35862–35871 (2019). https://doi.org/10.1039/c9ra02958c
Z. Zhou, F. Han, O.V. Prezhdo, Understanding divergent behaviors in the photocatalytic hydrogen evolution reaction on CdS and ZnS: a DFT based study. Phys. Chem. Chem. Phys. 18(25), 16862–16869 (2016). https://doi.org/10.1039/c6cp02599d
C.M. Marian, A. Heil, M. Kleinschmidt, The DFT/MRCI method. Wiley Interdiscip. Rev. Comput. Mol. Sci. (2019). https://doi.org/10.1002/wcms.1394
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
N. Mardirossian, M. Head-Gordon, ωb97X-V: a 10-parameter, range-separated hybrid, generalized gradient approximation density functional with nonlocal correlation, designed by a survival-of-the-fittest strategy. Phys. Chem. Chem. Phys. 16, 9904–9924 (2014). https://doi.org/10.1039/c3cp54374a
M.M. Obeid, H.R. Jappor, S.J. Edrees, M.M. Shukur, R. Khenata, Y. Mogulkoc, The electronic, half-metallic, and magnetic properties of Ca1–xCrxS ternary alloys: insights from the first-principle calculations. J. Mol. Graph. Model. 89, 22–32 (2019). https://doi.org/10.1016/j.jmgm.2019.02.004
M. Ernzerhof, G.E. Scuseria, Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 110, 5029–5036 (1999). https://doi.org/10.1063/1.478401
S. Chang, J. Li, Q. Yue, Z. Shao, Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field. Nanoscale Res. Lett. 8, 1–7 (2013)
S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006). https://doi.org/10.1002/jcc.20495
T. Chen, X. Meng, R. Jiang, J. Liang, Q. Yang, Q. Tan, C. Sun, X. Zhang, S. Ren, Electronic structure and optical properties of graphene/stanene heterobilayer. Phys. Chem. Chem. Phys. 18, 16302–16309 (2016). https://doi.org/10.1039/C6CP02424F
A. Hemeryck, A. Motta, C. Lacaze-Dufaure, D. Costa, P. Marcus, DFT-D study of adsorption of diaminoethane and propylamine molecules on anatase (101) TiO2 surface. Appl. Surf. Sci. 426, 107–115 (2017). https://doi.org/10.1016/j.apsusc.2017.07.161
P. Pyykkö, M. Atsumi, Molecular single-bond covalent radii for elements 1–118. Chem. A Eur. J. 15, 186–197 (2009). https://doi.org/10.1002/chem.200800987
X.-P. Yang, Q. Chen, J.-K. Meng, R.-S. Jiang, T.-L. Liang, Q.-H. Tan, C.-J. Cai, M. Sun, X. Yang, D.-G. Ren, First-principles study of sulfur dioxide sensor based on phosphorenes. IEEE Electron Device Lett. 37, 660–662 (2016). https://doi.org/10.1109/LED.2016.2543243
P.J.D. Segall, M.D. Lindan, C.J. Probert, M.J. Pickard, M.C. Hasnip, P.J. Clark, S.J. Payne, First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 14, 2717–2744 (2002). https://doi.org/10.1088/0953-8984/14/11/301
X. Obeid, M.M. Shukur, M.M. Edrees, S.J. Khenata, R. Ghebouli, M.A. Khandy, S.A. Bouhemadou, A. Jappor, H.R. Wang, Electronic band structure, thermodynamics and optical characteristics of BeO1−xAx (A = S, Se, Te) alloys: insights from ab initio study. Chem. Phys. 526, 110414 (2019). https://doi.org/10.1016/j.chemphys.2019.110414
X. Sun, X. Yang, Q. Meng, R. Tan, C. Liang, Q. Jiang, J. Ye, H. Chen, Adsorption of gas molecules on graphene-like InN monolayer: a first-principle study. Appl. Surf. Sci. 404, 291–299 (2017). https://doi.org/10.1016/j.apsusc.2017.01.264
A.R. Villegas, C.E.P. Rocha, Elucidating the optical properties of novel heterolayered materials based on MoTe2–InN for photovoltaic applications. J. Phys. Chem. C 119, 11886–11895 (2015). https://doi.org/10.1021/jp5122596
H.R. Jappor, Z.A. Saleh, M.A. Abdulsattar, Simulation of electronic structure of aluminum phosphide nanocrystals using ab initio large unit cell method. Adv. Mater. Sci. Eng. 2012, 1–6 (2012). https://doi.org/10.1155/2012/180679
A.H.A. Nasriya, Electronic properties calculation of CO and NO adsorbed on BN nanolayer, using density function theory DFT. J. Eng. Appl. Sci. 13, 89–96 (2018)
J.A. Shlaka, A.H. Abo Nasria, Density functional theory study on the adsorption of AsH3 gas molecule with monolayer (AlN)21 (including pristine, C, B doped and defective aluminium nitride sheet). IOP Conf. Ser. Mater. Sci. Eng. 928, 072082 (2020). https://doi.org/10.1088/1757-899X/928/7/072082
A.H.A. Nasriya, DFT study of COCL2 adsorption on pristine fullerence and doped fullerene. J. Eng. Appl. Sci. 13, 7507–7510 (2018)
H. Tributsch, Solar energy-assisted electrochemical splitting of water. Some energetical, kinetical and catalytical considerations verified on MoS2 layer crystal surfaces. Zeitschr. Naturforsch. A 32, 972–985 (1977). https://doi.org/10.1515/zna-1977-0911
A.N. Pikhtin, D.A. Yaskov, Infrared absorption in gallium phosphide. Phys. Stat. Solidi 34, 815–824 (1969). https://doi.org/10.1002/pssb.19690340244
M. Gajdoš, K. Hummer, G. Kresse, J. Furthmüller, F. Bechstedt, Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B Condens. Matter Mater. Phys. 73, 45112 (2006)
X. Sun, Y. Guo, Y. Zhao, S. Liu, H. Li, Gas adsorption investigation on sige monolayer: A first-principle calculation. Sensors (Switzerland) 20, 2879 (2020). https://doi.org/10.3390/s20102879
C. Yongqing, K. Qingqing, Z. Gang, Z. Yong-Wei, Energetics, charge transfer, and magnetism of small molecules physisorbed on phosphorene. J. Phys. Chem. C 119, 3102–3110 (2015). https://doi.org/10.1021/jp510863p
F.M. Berdiyorov, G.R. Neek-Amal, M. Hussein, I.A. Madjet, M.E. Peeters, Large CO2 uptake on a monolayer of CaO. J. Mater. Chem. A. 5, 2110–2114 (2017). https://doi.org/10.1039/c6ta08810d
P. Meng, R.-S. Cai, M. Jiang, J.-K. Liang, Q.-H. Sun, X. Yang, Q. Tan, C.-J. Chen, First principles investigation of small molecules adsorption on antimonene. IEEE Electron Device Lett. 38, 134–137 (2017). https://doi.org/10.1109/LED.2016.2633569
J. Jin, Y. Li, X. Yang, Single layer of MX 3 (M = Ti, Zr; X = S, Se, Te): a new platform for nano-electronics and optics. Phys. Chem. Chem. Phys. 17, 18665–18669 (2015). https://doi.org/10.1039/C5CP02813B
H.A. Abdulhussein, P. Ferrari, J. Vanbuel, C. Heard, A. Fielicke, P. Lievens, E. Janssens, R.L. Johnston, Altering CO binding on gold cluster cations by Pd-doping. Nanoscale 11, 16130–16141 (2019). https://doi.org/10.1039/C9NR04237G
P. Jensen, M. Spanner, P.R. Bunker, The CO2 molecule is never linear. J. Mol. Struct. 1212, 128087 (2020). https://doi.org/10.1016/j.molstruc.2020.128087
A. Föhlisch, M. Nyberg, J. Hasselström, O. Karis, L.G.M. Pettersson, A. Nilsson, How carbon monoxide adsorbs in different sites. Phys. Rev. Lett. 85, 3309–3312 (2000). https://doi.org/10.1103/PhysRevLett.85.3309
Y. Andersson, D.C. Langreth, B.I. Lundqvist, van der Waals interactions in density-functional theory. Phys. Rev. Lett. 76, 102–105 (1996). https://doi.org/10.1103/PhysRevLett.76.102
G.J. Jackson, J. Lüdecke, S.M. Driver, D.P. Woodruff, R.G. Jones, A. Chan, B.C.C. Cowie, The local adsorption structure of SO2 on Ni(111): a normal incidence X-ray standing wavefield determination. Surf. Sci. 389, 223–233 (1997). https://doi.org/10.1016/S0039-6028(97)00416-0
X. Liu, F. Sun, Z. Qu, J. Gao, S. Wu, The effect of functional groups on the SO2 adsorption on carbon surface I: a new insight into noncovalent interaction between SO2 molecule and acidic oxygen-containing groups. Appl. Surf. Sci. 369, 552–557 (2016). https://doi.org/10.1016/j.apsusc.2015.12.119
P. Nordlander, C. Holmberg, J. Harris, Physisorption interaction of H2 with simple metals. Surf. Sci. 175, L753–L758 (1986). https://doi.org/10.1016/0039-6028(86)90227-X
F. Frechard, P. Sautet, Chemisorption of H2 and H2S on the (100) surface of RuS2: an ab initio theoretical study. Surf. Sci. 389, 131–146 (1997). https://doi.org/10.1016/S0039-6028(97)00405-6
T.-T. Zhang, Q.-L. Tang, M.-Y. Yao, C. Chen, X.-X. Duan, Q. Wang, X. Zhang, M.-L. Zhang, W. Hu, Quantum chemical DFT study of molecular adsorption of H2S on clean and chemically modified Au(1 1 0) surfaces. Appl. Surf. Sci. 542, 148595 (2021). https://doi.org/10.1016/j.apsusc.2020.148595
Acknowledgements
The authors highly acknowledge the resources and technical support from the University of Kufa and the Ministry of Higher Education and Scientific Research of Iraq. All calculations have been performed on a processor PowerPC G5. The authors thank Dr. Nicola Seriani (the Abdus Salam ICTP, Italy) for the valuable discussion and helpful criticism on the manuscript.
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
AHAN designed the research project, supervised the work, and revised and edited the manuscript. WKA carried out the calculations and wrote the first draft of the manuscript. HAA validated the findings, administered the project, wrote the introduction, and revised and edited the manuscript. All authors contributed to data interpretation and discussion of the results.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Ethical approval
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alaarage, W.K., Abo Nasria, A.H. & Abdulhussein, H.A. Computational analysis of CdS monolayer nanosheets for gas-sensing applications. Eur. Phys. J. B 96, 134 (2023). https://doi.org/10.1140/epjb/s10051-023-00601-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjb/s10051-023-00601-3