Abstract
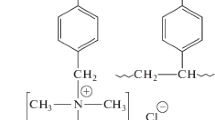
It has been found that after 300 h of operation of AMX and AMX-Sb anion-exchange membranes (Astom, Japan) in overlimiting current regimes in the process of electrodialysis desalination of 0.02 M NaCl, NH4Cl, NaH2PO4, and KC4H5O6 (KHT) solutions, the limiting currents, \(i_{{\lim }}^{{\exp }}\), determined by graphic processing of current–voltage curves increase in the order NaCl < NaH2PO4 < KHT. Their increments relative to those for the “fresh” membrane are 33, 90, and 128%, respectively. The growth in \(i_{{\lim }}^{{\exp }}\) is accompanied by an increase in the thickness of the samples occurring in the NaH2PO4 and KHT solutions. In the case of NH4Cl, the values of \(i_{{\lim }}^{{\exp }}\) decrease. It has been shown that a small decrease in counterion transport numbers during membrane operation has almost no effect on the values of limiting currents. The main contribution to the increase in \(i_{{\lim }}^{{\exp }}\) is apparently made by electroconvection, which develops according to the mechanism of electroosmosis of the first kind. Its development is facilitated by the growth in the number and size of free-standing micrometer-sized cavities on the surface of anion-exchange membranes, the area and linear dimensions of which increase in the order NaCl < NaH2PO4 < KHT. These cavities are formed as a result of enhancement of electrochemical degradation of the ion-exchange material and the inert filler polyvinyl chloride at the membrane/solution interface in ampholyte solutions.








Similar content being viewed by others
REFERENCES
L. Shi, W. S. Simplicio, G. Wu, et al., Curr. Pollut. Rep. 4 (2), 74–83.
A. Al-Mamun, W. Ahmad, M. S. Baawain, et al., J. Clean. Prod. 183, 458 (2018).
J. Ran, L. Wu, Y. He, et al., J. Membr. Sci. 522, 267 (2017).
C. R. Gally, T. Benvenuti, C. D. M. da Trindade, et al., J. Environ. Chem. Eng. 6, 5855 (2018).
S. Kazuhisa, J. Membr. Sci. 309, 175 (2008).
S. Galier and H. Roux-de Balmann, Sep. Purif. Technol. 77, 237 (2011).
H. Luo, X. Cheng, G. Liu, et al., J. Membr. Sci. 523, 122 (2017).
E. Husson, M. Araya-Farias, Y. Desjardins, and L. Bazinet, Innov. Food Sci. Emerging Technol. 17, 153 (2013).
X. Zhu and R. Bai, Curr. Pharm. Des. 23, 218 (2017).
W. Garcia-Vasquez, L. Dammak, C. Larchet, et al., J. Membr. Sci. 446, 255 (2013).
S. Suwal, J. Amiot, L. Beaulieu, and L. Bazinet, J. Membr. Sci. 510, 405 (2016).
V. Sarapulova, E. Nevakshenova, X. Nebavskaya, et al., J. Membr. Sci. 559, 170 (2018).
G. Merle, M. Wessling, and K. Nijmeijer, J. Membr. Sci. 377, 1 (2011).
Y. Mizutani, J. Membr. Sci. 49, 121 (1990).
J.-H. Choi and S.-H. Moon, J. Colloid Interface Sci. 265, 93 (2003).
S. A. Mareev, D. Yu. Butylskii, N. D. Pismenskaya, et al., J. Membr. Sci. 563, 768 (2018).
GOST (State Standard) 17553-72: Ion-Exchange Membranes: Methods for Preparation of Specimens for Tests (Izd. Standartov, Moscow, 1972) [in Russian].
N. D. Pismenskaya, E. E. Nevakshenova, and V. V. Nikonenko, Pet. Chem. 58, 465 (2018).
R. Lteif, L. Dammak, C. Larchet, and B. Auclair, Eur. Polym. J. 35, 1187 (1999).
V. I. Zabolotsky and V. V. Nikonenko, J. Membr. Sci. 79, 181 (1993).
C. Larchet, L. Dammak, B. Auclair, et al., New J. Chem. 28, 1260 (2004).
V. I. Zabolotskii and V. V. Nikonenko, Ion Transport in Membranes (Nauka, Moscow, 1996) [in Russian].
N. P. Gnusin, N. P. Berezina, A. A. Shudrenko, and O. P. Ivina, Zh. Fiz. Khim. 68, 565 (1994).
E. D. Belashova, O. A. Kharchenko, V. V. Sarapulova, et al. Pet. Chem. 57, 1145 (2017).
H.-W. Rösler, F. Maletzki, and E. A. Staude, J. Membr. Sci. 72, 171 (1992).
J. S. Newman, Electrochemical Systems (Prentice Hall, Englewood Cliffs, 1973), p. 309.
D. R. Lide, CRC Handbook of Chemistry and Physics (CRC, Boca Raton, 1995).
E. Korzhova, N. Pismenskaya, D. Lopatin, et al., J. Membr. Sci. 500, 161 (2016).
E. Kniaginicheva, N. Pismenskaya, S. Melnikov, et al., J. Membr. Sci. 496, 78 (2015).
N. D. Pismenskaya, E. V. Pokhidnia, G. Pourcelly, and V. V. Nikonenko, J. Membr. Sci. 566, 54 (2018).
P. E. Mason, J. M. Cruickshank, G. W. Neilson, and P. Buchanan, PhysChemChemPhys. 5, 4686 (2003).
Y. Koga, T. Kondo, Y. Miyazaki, and A. Inaba, J. Solution Chem. 41, 1388 (2012).
F. G. Helfferich, Ion Exchange (McGraw-Hill, New York, 1962).
V. Sarapulova, E. Nevakshenova, N. Pismenskaya, L. Dammak, V. Nikonenko, J. Membr. Sci. 479, 28–38 (2015).
V. V. Nikonenko, S. A. Mareev, N. D. Pis’menskaya, et al., Russ. J. Electrochem. 53, 1122 (2017).
N. A. Mishchuk, Adv. Colloid Interface Sci. 160, 16 (2010).
I. Rubinstein and B. Zaltzman, Phys. Rev. Lett. 114, 114502 (2015).
K. A. Nebavskaya, V. V. Sarapulova, K. G. Sabbatovskiy, et al., J. Membr. Sci. 523, 36 (2017).
N. D. Pismenskaya, V. V. Nikonenko, N. A. Melnik, et al., J. Phys. Chem. B 116, 2145 (2012).
K. F. Hagesteijn, S. Jiang, and B. P. Ladewig, J. Mater. Sci. 53, 11131 (2018).
R. Simons, Electrochim. Acta 29, 151 (1984).
S. H. Pine Org. React. 18, 403 (1970).
O. A. Kozaderova, S. I. Niftaliev, and K. B. Kim, Russ. J. Electrochem, 54, 363 (2018).
V. I. Zabolotskii, N. V. Shel’deshov, and N. P. Gnusin, Russ. Chem. Rev. 57, 801 (1988).
T. Sata, M. Tsujimoto, T. Yamaguchi, and K. Matsusaki, J. Membr. Sci. 112, 161 (1996).
K. S. Minsker and G. T. Fedoseeva, Degradation and Stabilization of Polyvinyl Chloride, 2nd Ed. (Khimiya, Moscow, 1979) [in Russian].
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Basic Research, project no. 16-48-230852 reg_a. The authors thank the scientific and educational environmental–analytical center at the Kuban State University (unique project identifier RFMEFI59317Х0008) for providing scientific equipment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Rybalkina, O.A., Tsygurina, K.A., Sarapulova, V.V. et al. Evolution of Current–Voltage Characteristics and Surface Morphology of Homogeneous Anion-Exchange Membranes during the Electrodialysis Desalination of Alkali Metal Salt Solutions. Membr. Membr. Technol. 1, 107–119 (2019). https://doi.org/10.1134/S2517751619020094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2517751619020094