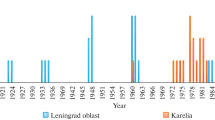
Abstract
The role of parasites (parasitoids and infections) in the population dynamics of the gypsy moth (Lymantria dispar L.) and the nun moth (Lymantria monacha L.), which are prone to large-scale and panzonal periodic outbreaks, is discussed. The results of long-term research have shown that parasites are not the key regulators of the abundance of forest phytophagous insects with high biotic potential, even though they reduce its values, mainly because of the elimination of specimens with low viability. Outbreaks of these parasites are explained by the influence of stress abiotic factors (for example, of droughts) on cenopopulations of woody plants rather than by a delay in parasitic activity or insect escape. Plant reactions to stress under certain biotopic conditions cause changes in the biochemical composition of leaves and needles that are favorable for phyllophages with high biotic potential and rapid adaptation to new environmental and climatic conditions. For this reason, in the system of tree-phytophage interactions, the trophic factor is of primary importance. This role in the phytophage-parasite system is indirectly associated with a sharp decline in the immunity of insects and their increased sensitivity to parasites. It should be noted that this assumption is significant only for forest phytophagous insects having high biotic potential, which are subjected to large-scale and panzonal periodic outbreaks of their abundance.
Similar content being viewed by others
References
Abbott, K.C. and Dwyer, G., Food limitation and insect outbreaks: complex dynamics in plant-herbivore models, J. Anim. Ecol., 2007, vol. 76, no. 5, pp. 1004–1014.
Agrawal, A.A., Lau, J.A., and Hamback, P.A., Community heterogeneity and the evolution of interactions between plants and insect herbivores, Quart. Rev. Biol., 2006, vol. 81, no. 4, pp. 349–376.
Ahmad, S. and Pardini, R.S., Mechanisms for regulating oxygen toxicity in phytophagous insects, Free Radic. Biol. Med., 1990, vol. 8(4), pp. 401–413.
Bakhvalov, S.A., Influence of relationships in the system plant-insect-parasite on development and population dynamics of insects, Extended Abstracts of Doctoral (Biol.) Dissertation, Novosibirsk, 2008.
Bakhvalov, S.A., Bakhvalova, V.N., and Martem’yanov, V.V., Role of trophic factor in insect population dynamic: problem analysis, Usp. Sovrem. Biol., 2006, vol. 126, no. 1, pp. 49–60.
Bakhvalov, S.A., Bashev, A.N., and Knor, I.B., Dynamics of populations of black arches Lymantria monacha L. (Lymantriidae: Lepidoptera) and its parasite baculovirus in Western Siberia, Lesovedenie, 1998, no. 4, pp. 26–33.
Bakhvalov, S.A., Il’inykh, A.V., Zhimerikhin, V.N., and Martem’yanov, V.V., Dynamics of black arches Lymantria monacha L. and gypsy moth L. dispar L. (Lymantriidae, Lepidoptera): role of food resources and virus infection, Evraz. Entomol. Zh., 2002, vol. 1, no. 1, pp. 101–108.
Bakhvalov, S.A., Koltunov, E.V., and Martem’yanov, V.V. Faktory i ekologicheskie mekhanizmy populyatsionnoi dinamiki lesnykh nasekomykh-fillofagov (Factors and Environmental Mechanisms of Population Dynamics of Forest Insects-Phyllophages), Novosibirsk: Nauka, 2010.
Battisti, A., Host-plant relationships and population dynamics of the pine processionary caterpillar Thaumetopoea pityocampa (Denis & Schiffermuller), Z. Angew. Entomol., 1988, vol. 105, no. 4, pp. 393–402.
Beninger, C.W., Abou-Zaid, M.M., Kinstner, A.L.E., et al., A flavanone and two phenolic acids from Chrysanthemum morifolium with phytotoxic and insect growth regulating activity, J. Chem. Ecol., 2004, vol. 30, no. 3, pp. 589–606.
Bernays, E.A. and Chapman, R.F., Plant secondary compounds and grasshoppers: beyond plant defenses, J. Chem. Ecol., 2000, vol. 26, pp. 1773–1794.
Bonsall, M.B., van der Meijden, E., and Crawley, M.J., Contrasting dynamics in the same plant-herbivore interaction, Proc. Natl. Acad. Sci. U.S.A., 2003, vol. 100, no. 25, pp. 1493–1498.
Bruinsma, M. and Dicke, M., Herbivore-induced indirect defense: from induction mechanisms to community ecology, in Induced Plant Resistance to Herbivory, Springer, 2008, pp. 31–60.
Bryant, J.P., Heitkonig, I. Kuropat, P., and Owen-Smith, N., Effects of severe defoliation on the long-term resistance to insect attack and on leaf chemistry in six woody species of the southern African savanna, Am. Nat., 1991, vol. 137, pp. 50–63.
Buntgen, U., Frank, D., Liebhold, A., et al., Three centuries of insect outbreaks across, Eur. Alps New Phytol., 2009, vol. 182, no. 4, pp. 929–941.
Byington, T.S., Gottschalk, K.W., and Mcgraw, J.B., Within—population variation in response of red oak seedlings to herbivory by gypsy moth larvae, Am. Midl. Nat., 1994, vol. 132, no. 2, pp. 328–339.
Cai, Q.N, Ma, X.M, Zhao, X., et al., Effects of host plant resistance on insect pests and its parasitoid: a case study of wheat-aphid-parasitoid system, Biol. Control, 2009, vol. 49, no. 2, pp. 134–138.
Chernyshev, V.B., Ekologiya nasekomykh (Ecology of the Insects), Moscow: Mosk. Gos. Univ., 1996.
Classen, A.T., Chapman, S.K., Whitham, T.G., et al., Genetic based plant resistance and susceptibility traits to herbivory influence needle and root litter nutrient dynamics, J. Ecol., 2007, vol. 95, no. 6, pp. 1181–1194.
Cory, J.S. and Hoover, K., Plant-mediated effects in insect-pathogen interactions, Trends Ecol. Evol., 2006, vol. 21, pp. 278–286.
Crone, E.E. and Jones, C.G., The dynamics of carbon-nutrient balance: effects of cottonwood to short- and long-term shade on beetle feeding preferences, J. Chem. Ecol., 1999, vol. 25, no. 3, pp. 635–656.
Cuevas-Reyes, P., Quesada, M., Hanson, P., and Oyama, K., Interactions among three trophic levels and diversity of parasitoids: a case of top-down processes in Mexican tropical dry forest, Environ. Entomol., 2007, vol. 36, no. 4, pp. 792–800.
Dalin, P., Habitat difference in abundance of willow leaf beetle Phratora vulgatissima (Coleoptera: Chrysomelidae): plant quality or natural enemies? Bull. Entomol. Res., 2006, vol. 96, no. 6, pp. 629–635.
Damico, V., Elkinton, J.S. Dwyer, G., Willis, R.B., and Montgomery, M.E., Foliage damage does not affect within-season transmission of an insect virus, Ecology, 1998, vol. 79, no. 3, pp. 1104–1110.
Denno, R.F., Lewis, D., and Gratton, C., Spatial variation in the relative strength of top-down and bottom-up forces: causes and consequences for phytophagous insect populations, Ann. Zool. Fenn., 2005, vol. 42, no. 4, pp. 295–311.
Donaldson, J.R., Kruger, E.L., and Lindroth, R.L., Competition- and resource-mediated tradeoffs between growth and defensive chemistry in trembling aspen (Populus tremuloides), New Phytol., 2006, vol. 169, no. 3, pp. 561–570.
Dwyer, G., Firestone, J., and Stevens, T.E., Should models of disease dynamics in herbivorous insects include the effects of variability in host plant foliage quality? Am. Nat., 2005, vol. 165, pp. 16–31.
Dynamics of Forest Insect Populations, Berryman, A.A., Ed., New York: Plenum Press, 1988.
Felton, G.W. and Gtehouse, J.A., Antinutritive plant defense mechanisms, in Biology of the Insect Midgut, Lehane, M.J. and Billingsley, P.F., Eds., London: Chapman and Hall, 1996, pp. 373–416.
Fleder, W., Waldbauliche Aspecte im Zusammenhang mit Kalamitaten durch “Freifressende Schmetterlingsraupen im Forst,” in Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft, Berlin: Dahlem, 1994, vol. 293, pp. 82–93.
Gandhi, K.J.K. and Herms, D.A., Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America, Biol. Invasions, 2010, vol. 12, no. 2, pp. 389–405.
Gaylord, E.S., Preszler, R.W., and Boecklen, W.J., Interactions between host plants, endophytic fungi and a phytophagous insect in an oak (Quercus griseax, Q. gambelii) hybrid zone, Oecologia, 1996, vol. 105, no. 3, pp. 336–342.
Georgievska, L., Joosten, N., Hoover, K., et al., Effects of single and mixed infections with wild type and genetically modified Helicoverpa armigera nucleopolyhedrovirus on movement behavior of cotton bollworm larvae, Auth. Entomol. Exp. Appl., 2010, vol. 135, pp. 56–67.
Giertych, M.J., Bakowski, M., Karolewski, P., et al., Influence of mineral fertilization on food quality of oak leaves and utilization efficiency of food components by the gypsy moth, Entomol. Exp. Appl., 2005, vol. 117, no. 1, pp. 59–69.
Giertych, M.J., Karolewski, P., Grzebyta, J., and Oleksyn, J., Feeding behavior and performance of Neodiprion sertifer larvae reared on Pinus sylvestris needles, For. Ecol. Manage., 2007, vol. 242, nos. 2–3, pp. 700–707.
Glupov, V.V. and Bakhvalov, S.A., Resistance mechanisms of the insects at pathogenesis, Usp. Sovrem. Biol., 1998, vol. 118, no. 4, pp. 466–482.
Gruppe, A., Die Bedeutung von Nadelinhaltsstoffen fur den Entwicklungserfolg von Nonen-Larven (Lymantria monacha L.), Mitt. Dtsch. Ges. Allg. Angew. Entomol., 1993, vol. 8, nos. 4–6, pp. 497–498.
Haack, R.A. and Mattson, W.J., They nibbled while the forests burned, Nat. Hist., 1989, vol. 98, no. 1, pp. 56–57.
Harrison, S., Persistent, localized outbreaks in the western tussock moth Orgyia vetusta: the roles of resource quality, predation and poor dispersal, Ecol. Entomol., 1997, vol. 22, no. 2, pp. 158–166.
Harvey, J.A., van Nouhuys, S., and Biere, A., Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids, J. Chem. Ecol., 2005, vol. 31, no. 2, pp. 287–302.
Haukioja, E., Induction of defense in trees, Ann. Rev. Entomol. (Palo Alto, Calif.), 1991, pp. 25–42.
Haukioja, E., Nutritive quality as a defense against herbivores, in Proc. 18th Int. Congr. Entomol., Abstracts and Author Index, Vancouver, 1988, p. 421.
Haukioja, E., Plant defenses and population fluctuations of forest defoliators: mechanism-based scenarios, Ann. Zool. Fennici., 2005, vol. 42, pp. 313–325.
Haukioja, E., Putting the insect into the birch-insect interaction, Oecologia, 2003, vol. 136, pp. 161–168.
Hausmann, C., Wackers, F.L., and Dorn, S., Sugar convertibility in the parasitoid Cotesia glomerata (Hymenoptera: Braconidae), Arch. Insect Biochem. Physiol., 2005, vol. 60, no. 4, pp. 223–229.
Helms, S.E. and Hunter, M.D., Variation in plant quality and the population dynamics of herbivores: there is nothing average about aphids, Oecologia, 2005, vol. 145, no. 2, pp. 197–204.
Hemming, J.D.C. and Lindroth, R.L., Intraspecific variation in aspen phytochemistry: effects on performance of gypsy moth and forest tent caterpillars, Oecologia, 1995, vol. 103, no. 1, pp. 79–88.
Herniou, E.A. and Jehle, J.A., Baculovirus phylogeny and evolution, Curr. Drug Targets, 2007, vol. 8, no. 10, pp. 1043–1050.
Hogstedt, G., Seldal, T., and Breistol, A., Period length in cyclic animal populations, Ecology, 2005, vol. 86, pp. 373–378.
Hunter, A.F., Ecology, life history, and phylogeny of outbreak and nonoutbreak species, in Population Dynamics: New Approaches and Synthesis, Cappucino, N. and Price, P.W., Åds., New York: Academic Press, 1995, pp. 41–64.
Hunter, M.D., Multiple approaches to estimating the relative importance of top-down and bottom-up forces on insect populations: experiments, life tables and time series analysis, Basic Appl. Ecol., 2001, vol. 2, no. 4, pp. 295–309.
Insect Outbreaks, Barbosa, P. and Schultz, J.C., Eds., London: Academic, 1987.
Isaev, A.S., Khlebopros, R.G., Nedorezov, L.V., et al., Dinamika chislennosti lesnykh nasekomykh (Population Dynamics of Forest Insects), Novosibirsk: Nauka, 1984.
Ivashchenko, L.S., Role of the plants in interaction with the insects, in Zashchishchennost’ rastitel’nosti v usloviyakh reformirovaniya agropromyshlennogo kompleksa (Protection of Vegetation in Conditions of Reforms in Agricultural Complex), St. Petersburg, 1995, pp. 198–199.
Johnson, M.T.J., Bottom-up effects of plant genotype on aphids, ants, and predators, Ecology, 2008, vol. 89, no. 1, pp. 145–154.
Kaitaniemi, P. and Ruohomaki, K., Sources of variability in plant resistance against insects: free caterpillars show strongest effects, Oikos, 2001, vol. 95, no. 3, pp. 461–470.
Kaitaniemi, P., Ruohomaki, K., Ossipov, V., et al., Delayed induced changes in the biochemical composition of host plant leaves during an insect outbreak, Oecologia, 1998, vol. 116, nos. 1–2, pp. 182–190.
Kendall, B.E., Ellner, S.P., McCauley, E., et al., Population cycles in the pine looper moth: dynamical tests of mechanistic hypotheses, Ecol. Monogr., 2005, vol. 75, pp. 259–276.
Knape, J. and de Valpine, P., Effects of weather and climate on the dynamics of animal population time series, Proc. R. Soc. B., 2011, vol. 278, no. 1708, pp. 985–992.
Koltunov, E.V., Nasekomye-fitofagi lesnykh biogeotsenozov v usloviyakh antropogennogo vozdeistviya (Insects-Phytophages in Forest Biogeocenosises Affected Anthropogenic Factors), Yekaterinburg: Nauka, 1993.
Koltunov, E.V., Ekologiya neparnogo shelkopryada v lesakh Evrazii (Ecology of Gypsy Moth in Eurasian Forests), Yekaterinburg: UrO, Ross. Akad. Nauk, 2006.
Koltunov, E.V. and Khamidullina, M.I., The reasons of mass reproduction burst of the gypsy moth in Trans-Ural forest-steppe, Agro XXI, 2009, nos. 1–3, pp. 23–25.
Koltunov, E.V. and Khamidullina, M.I., Influence of defoliation on concentration of phenol-containing compounds in the leaves of silver birch (Betula pendula Roth.) affected by anthropogenic factors, Sovrem. Probl. Nauki Obraz., 2012, no. 6. http://www.science-education.ru/106-7436
Koltunov, E.V., Ponomarev, V.I., and Fedorenko, S.I., Ekologiya neparnogo shelkopryada v usloviyakh antropogennogo vozdeistviya (Ecology of Gypsy Moth Affected by Anthropogenic Factors), Yekaterinburg: Inst. Lesa, 1998.
Kondakov, Yu.P., Mass reproduction of Siberian silk moth in the forests of Krasnoyarsk krai, Entomol. Issled. Sibiri, 2002, no. 2, pp. 25–74.
Konikov, A.S., On biocoenotic and population levels of insect’s adaptation to habitat conditions, Ekologiya, 1971, no. 1, pp. 80–86.
Konikov, A.S., Regulyatory chislennosti lesnykh nasekomykh (Regulating Factors of Forest Insect Population Number), Novosibirsk: Nauka, 1978.
Le Mellec, A., Gerold, G., and Michalzik, B., Insect herbivory, organic matter deposition and effects on below-ground organic matter fluxes in a central European oak forest, Plant Soil, 2011, vol. 342, nos. 1–2, pp. 393–403.
Liebhold, A.M. and Tobin, P.C., Population ecology of insect invasions and their management, Annu. Rev. Entomol., 2008, vol. 53, pp. 387–408.
Marques, E.S.D., Price, P.W., and Cobb, N.S., Resource abundance and insect herbivore diversity on woody Fabaceous desert plants, Environ. Entomol., 2000, vol. 29, no. 4, pp. 696–703.
Martem’yanov, V.V. and Bakhvalov, S.A., Environmental relationships in the system of triotrophic and their influence on development and population dynamics of forest phyllophages, Evroaziat. Entomol. Zh., 2007, vol. 6, no. 2, pp. 205–221.
Mattson, W.J., Haack, R.H., Lawrence, R.K., and Slocum, S.S., Considering the nutritional ecology of the spruce budworm in its management, For. Ecol. Manag., 1991, vol. 39, nos. 1–4, pp. 183–210.
McMillin, J.D. and Wagner, M.R., Chronic defoliation impacts pine sawfly (Hymenoptera: Diprionidae) performance and host plant quality, Oikos, 1997, vol. 79, no. 2, pp. 357–362.
Meade, T., Felton, G.W., and Young, S.Y., Interactions among plants, herbivores, and entomopathogens: implications for pest management, in XIII Int. Plant Protection Conf., The Hague, 1995, p. 435.
Morales, M.A. and Beal, A.L.H., Effects of host plant quality and ant tending for treehopper Publilia concava, Ann. Entomol. Soc. Am., 2006, vol. 99, no. 3, pp. 545–552.
Moran, P.J., Plant-mediated interactions between insects and a fungal plant pathogen and the role of plant chemical responses to infection, Oecologia, 1998, vol. 115, no. 4, pp. 523–530.
Nadzor, uchet i prognoz massovykh razmnozhenii khvoe- i listogryzushchikh nasekomykh v lesakh SSSR (Control, Calculation, and Forecast of Mass Reproduction of the Needle- and Leaf Beetles in USSR Forest), Il’inskii, A.I. and Tropin, I.V., Eds., Moscow: Lesn. Prom-st., 1965.
Nakamura, M., Miyamoto, Y., and Ohgushi, T., Gall initiation enhances the availability of food resources for herbivorous insects, Funct. Ecol., 2003, vol. 17, no. 6, pp. 851–857.
Navon, A., Hare, J.D., and Federici, B.A., Interactions among Heliothis virescens larvae, cotton condensed tannin and the cryia (c) delta—endotoxin of Bacillus thuringiensis, J. Chem. Ecol., 1993, vol. 19, no. 11, pp. 2485–2499.
Osier, T.L. and Lindroth, R.L., Long-term effects of defoliation on quaking aspen in relation to genotype and nutrient availability: plant growth, phytochemistry and insect performance, Oecologia, 2004, vol. 139, no. 1, pp. 55–65.
Parry, D., Herms, D.A., and Mattson, W.J., Responses of an insect folivore and its parasitoids to multiyear experimental defoliation of aspen, Ecology, 2003, vol. 84, no. 7, pp. 1768–1783.
Pasqualone, A.A. and Davis, J.M., The use of conspecific phenotypic states as information during reproductive decisions, Anim. Behav., 2011, vol. 82, no. 2, pp. 281–284.
Piubelli, G.C., Hoffmann-Campo, C.B., Moscardi, F., et al., Baculovirus-resistant Anticarsia gemmatalis responds differently to dietary rutin, Entomol. Exp. Appl., 2006, vol. 119, no. 1, pp. 53–60.
Pokozii, I.T., Aleksenitser, M.L., Bereznitskaya, N.N., et al., Some physiological reactions of the Chinese oak silkworm (Antheraea pernyi G.M) feeding with conserve food, Izv. Khar. Entomol. O-va, 1994, vol. 2, no. 1, pp. 108–115.
Rafes, P.M., Biogeocenologic theory of population dynamics of herbivore forest insects, in Matematicheskoe modelirovanie v ekologii (Mathematical Modeling in Ecology), Moscow: Nauka, 1978, pp. 34–51.
Rafes, P.M., Interaction of leaf-damaging forest insects and the trees, Entomologiya, 1981, vol. 5, pp. 140–202.
Rafes, P.M., Mass reproduction of leaf beetles as the disease of forest biogeocenosises, Byull. Mosk. O-va. Ispyt. Prir., Otd. Biol., 1989, vol. 94, no. 4, pp. 3–14.
Raymond, B., Hartley, S.E., Cory, J.S., and Hails, R.S., The role of food plant and pathogen-induced behavior in the persistence of a nucleopolyhedrovirus, J. Invertebr. Pathol., 2005, vol. 88, no. 1, pp. 49–57.
Regniere, J. and Nealis, V.G., Ecological mechanisms of population change during outbreaks of the spruce budworm, Ecol. Entomol., 2007, vol. 32, no. 5, pp. 461–477.
Reilly, J.R. and Hajek, A.E., Density-dependent resistance of the gypsy moth Lymantria dispar to its nucleopolyhedrovirus, and the consequences for population dynamics, Oecologia, 2008, vol. 154, no. 4, pp. 691–701.
Rudnev, D.F., Influence of physiological conditions of the plants in mass reproduction of forest insects-parasites, Zool. Zh., 1962, vol. 41, no. 3, pp. 313–329.
Schowalter, T.D., Hardrowe, W.W., and Crossley, D.A., Herbivory in forested ecosystems, Annu. Rev. Entomol., 1986, vol. 31, pp. 177–196.
Schwerdtfeger, F., Is the density of animal populations regulated by mechanisms or by chance? Int. Congr. Entomol. Proc., 1958, vol. 4, pp. 115–152.
Scriber, J.M., Integrating ancient patterns and current dynamics of insect-plant interactions: taxonomic and geographic variation in herbivore insect specialization, Science, 2010, vol. 17, no. 6, pp. 471–507.
Shapiro, M., Robertson, J.L., and Webb, R.E., Effect of nemm seed extract upon the gypsy moth (Lepidoptera, Lymantriidae) and its nuclear polyhedrosis virus, J. Econ. Entomol., 1994, vol. 87, no. 2, pp. 356–360.
Stadnitskii, G.V., Introduction to the general theory of forest protection, in Ekologiya i zashchita lesa (Ecology and Forest Protection), Leningrad, 1988, pp. 87–91.
Tonnang, H.E.Z., Nedorezov, L.V., Owino, J.O., and Ochanda Lohr, B., Host-parasitoid population density prediction using artificial neural networks: diamondback moth and its natural enemies, Agric. For. Entomol., 2010, vol. 12, no. 3, pp. 233–242.
Tooker, J.F. and Hanks, L.M., Tritrophic interactions and reproductive fitness of the prairie perennial Silphium laciniatum Gillette (Asteraceae), Environ. Entomol., 2006, vol. 35, no. 2, pp. 537–545.
Treutter, D., Biosynthesis of phenolic compounds and its regulation in apple, Plant Growth Regul., 2001, vol. 34, pp. 71–89.
Turchin, P., Wood, S.N., Ellner, S.P., et al., Dynamical effects of plant quality and parasitism on population cycles of larch bud moth, Ecology, 2003, vol. 84, no. 5, pp. 1207–1214.
Viktorov, G.A., Problema dinamiki chislennosti nasekomykh na primere vrednoi cherepashki (Problems of Mass Reproduction of the Insects by Example of the Sunn Pest), Moscow: Nauka, 1967.
Watt, A.D., Leather, S.R., and Forrest, G.I., The effect of previous defoliation of pole—stage lodgepole pine on plant chemistry and on the growth and survival of pine beauly moth (Panolis flammea) larvae, Oecologia, 1991, vol. 86, no. 1, pp. 31–35.
Willis, A.J., Thomas, M.B., and Lawton, J.H., Is the increased vigour of invasive weeds explained by a trade—off between growth and herbivore resistance? Oecologia, 1999, vol. 120, no. 4, pp. 632–640.
Winkler, K., Wackers, F., Bukovinszkine-Kiss, G., and van Lenteren, J., Sugar resources are vital for Diadegma semiclausum fecundity under field conditions, Basic Appl. Ecol., 2006, vol. 7, no. 2, pp. 133–140.
Zaprometov, M.N., Fenol’nye soedineniya: rasprostranenie, metabolism, i funktsii v rasteniyakh (Plant Phenolic Compounds: Distribution, Metabolism, and Functions), Moscow: Nauka, 1993.
Zvereva, E.L., Kozlov, M.V., Niemela, P., and Haukioja, E., Delayed induced resistance and increase in leaf fluctuating asymmetry as responses of Salix borealis to insect herbivory, Oecologia, 1997, vol. 109, no. 3, pp. 368–373.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Koltunov, S.A. Bakhvalov, V.N. Bakhvalova, V.N. Zhimerikin, 2014, published in Uspekhi Sovremennoi Biologii, 2014, Vol. 134, No. 3, pp. 270–284.
Rights and permissions
About this article
Cite this article
Koltunov, E.V., Bakhvalov, S.A., Bakhvalova, V.N. et al. The role of parasitoids and virus infections in the population dynamics of mass species of insects-phyllophages. Biol Bull Rev 4, 484–495 (2014). https://doi.org/10.1134/S2079086414060048
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086414060048