Abstract
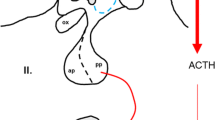
The transgenic mouse technology is widespread in the analysis of physiological functions; however, until now 22.0% of the tested null mutations were found to be lethal. The complete lack of vasopressin (AVP) also resulted in preweaning lethality. It is surprising to take into consideration the viability of the AVP natural mutant Brattleboro rats. Thus, AVP is crucial for survival, but it remains unclear which of its different roles is the most important. AVP exerts its effect through specific plasma membrane receptors. The V1a receptors can induce vasoconstriction maintaining blood pressure during hypovolaemia. The V1b receptor on the anterior pituitary has a role in stress adaptation. The V2 subtype is located in the kidney and contributes to the antidiuresis. The avp gene consists of a signal peptide, AVP, neurophysin 2 and a C-terminal glycopeptide. The naturally occurring AVP-deficient Brattleboro rat has a framshift mutation in the neurophysin portion resulting in central insipidus diabetes. In its hypothalamus AVP is not produced, while in certain peripheral tissues, it may be expressed, suggesting the existence of a different synthetic pathway. The avp knockout mice can also be produced; they will be born, but without the peripheral AVP administration, they will not survive. Comparing the available knockout models, we can conclude that the combined V1a and V2 receptormediated effects, namely, hypotension and water loss, may together led to lethality. As in the Brattleboro and targeted knockout mice the local synthesis of AVP in the heart can be maintained and AVP can be released into the general circulation. Thus, in these animals vasoconstriction can compensate the hypovolemia.
Similar content being viewed by others
References
Babey, M., Kopp, P., and Robertson, G.L., Familial forms of diabetes insipidus: clinical and molecular characteristics, Nat. Rev. Endocrinol., 2011, vol. 7, pp. 701–714.
Bielsky, I.F., Hu, S.B., Szegda, K.L., Westphal, H., and Young, L.J., Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice, Neuropsychopharmacology, 2004, vol. 29, pp. 483–493.
Carrazana, E.J., Pasieka, K.B., and Majzoub, J.A., The vasopressin mRNA poly(A) tract is unusually long and increases during stimulation of vasopressin gene expression in vivo, Mol. Cell. Biol., 1988, vol. 8, pp. 2267–2274.
Chen, J. and Aguilera, G., Vasopressin protects hippocampal neurones in culture against nutrient deprivation or glutamate- induced apoptosis, J. Neuroendocrinol., 2010, vol. 22, pp. 1072–1081.
Chen, Q., Patel, R., Sales, A., Oji, G., Kim, J., Monreal, A.W., and Brinton, R.D., Vasopressin-induced neurotrophism in cultured neurons of the cerebral cortex: Dependency on calcium signaling and protein kinase C activity, Neuroscience, 2000, vol. 101, pp. 19–26.
Egashira, N., Tanoue, A., Higashihara, F., Mishima, K., Fukue, Y., Takano, Y., Tsujimoto, G., Iwasaki, K., and Fujiwara, M., V1a receptor knockout mice exhibit impairment of spatial memory in an eight-arm radial maze, Neurosci. Lett., 2004, vol. 356, pp. 195–198.
Ermisch, A., Ruhle, H.J., Landgraf, R., and Hess, J., Blood-brain barrier and peptides, J. Cerebr. Blood Flow Metab., 1985, vol. 5, pp. 350–357.
Forti, F.L. and Armelin, H.A., Arginine vasopressin controls p27(Kip1) protein expression by PKC activation and irreversibly inhibits the proliferation of K-Ras-dependent mouse Y1 adrenocortical malignant cells, Biochim. Biophys. Acta, 2011, vol. 1813, pp. 1438–1445.
Friedmann, A.S., Memoli, V.A., Cheng, S.W., Yu, X., and North, W.G., Vasopressin and vasopressin-associated neurophysin are present in gastric and duodenal cells of Brattleboro and Long–Evans rats, Ann. N. Y. Acad. Sci., 1993a, vol. 689, pp. 522–525.
Friedmann, A.S., Memoli, V.A., Yu, X.M., and North, W.G., Biosynthesis of vasopressin by gastrointestinal cells of Brattleboro and Long–Evans rats, Peptides, 1993b, vol. 14, pp. 607–612.
Furuse, M., Knockout animals and natural mutations as experimental and diagnostic tool for studying tight junction functions in vivo, Biochim. Biophys. Acta, 2009, vol. 1788, pp. 813–819.
Ganong, W.F., Neuropeptides in cardiovascular control, J. Hypertension, 1984, vol. 2, pp. S15–S23.
Grant, F.D., Reventos, J., Kawabata, S., Miller, M., Gordon, J.W., and Majzoub, J.A., Transgenic mouse models of vasopressin expression, Hypertension, 1993, vol. 22, pp. 640–645.
Habener, J.F., Cwikel, B.J., Hermann, H., Hammer, R.E., Palmiter, R.D., and Brinster, R.L., Metallothionein-vasopressin fusion gene expression in transgenic mice. Nephrogenic diabetes insipidus and brain transcripts localized to magnocellular neurons, J. Biol. Chem., 1989, vol. 264, pp. 18844–18852.
Hanahan, D., Wagner, E.F., and Palmiter, R.D., The origins of oncomice: A history of the first transgenic mice genetically engineered to develop cancer, Genes Dev., 2007, vol. 21, pp. 2258–2270.
Hiroyama, M., Wang, S., Aoyagi, T., Oikawa, R., Sanbe, A., Takeo, S., and Tanoue, A., Vasopressin promotes cardiomyocyte hypertrophy via the vasopressin V1A receptor in neonatal mice, Eur. J. Pharmacol., 2007, vol. 559, pp. 89–97.
Hosoda, K., Hammer, R.E., Richardson, J.A., Baynash, A.G., Cheung, J.C., Giaid, A., and Yanagisawa, M., Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice, Cell, 1994, vol. 79, pp. 1267–1276.
Hupf, H., Grimm, D., Riegger, G.A., and Schunkert, H., Evidence for a vasopressin system in the rat heart, Circ. Res., 1999, vol. 84, pp. 365–370.
Ivell, R., Schmale, H., Krisch, B., Nahke, P., and Richter, D., Expression of a mutant vasopressin gene: Differential polyadenylation and readthrough of the mRNA 3' end in a frame-shift mutant, EMBO J., 1986, vol. 5, pp. 971–977.
Iwasaki, Y., Oiso, Y., Saito, H., and Majzoub, J.A., Effects of various mutations in the neurophysin/glycopeptide portion of the vasopressin gene on vasopressin expression in vitro, Tohoku J. Exp. Med., 2000, vol. 191, pp. 187–202.
Izumi, Y., Miura, K., and Iwao, H., Therapeutic potential of vasopressin-receptor antagonists in heart failure, J. Pharmacol. Sci., 2014, vol. 124, pp. 1–6.
Koufaris, C., Alexandrou, A., Sismani, C., and Skordis, N., Identification of an AVP-NPII mutation within the AVP moiety in a family with neurohypophyseal diabetes insipidus: Review of the literature, Hormones, 2015, vol. 14, pp. 442–446.
Landgraf, R. and Wigger, A., Born to be anxious: Neuroendocrine and genetic correlates of trait anxiety in HAB rats, Stress, 2003, vol. 6, pp. 111–119.
Landgraf, R., Kessler, M.S., Bunck, M., Murgatroyd, C., Spengler, D., Zimbelmann, M., Nussbaumer, M., Czibere, L., Turck, C.W., Singewald, N., Rujescu, D., and Frank, E., Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: Focus on vasopressin and glyoxalase-I, Neurosci. Biobehav. Rev., 2007, vol. 31, pp. 89–102.
Lim, A.T., Lolait, S.J., Barlow, J.W., Autelitano, D.J., Toh, B.H., Boublik, J., Abraham, J., Johnston, C.I., and Funder, J.W., Immunoreactive argininevasopressin in Brattleboro rat ovary, Nature, 1984, vol. 310, pp. 61–64.
Majzoub, J.A., Carrazana, E.J., Shulman, J.S., Baker, K.J., and Emanuel, R.L., Defective regulation of vasopressin gene expression in Brattleboro rats, Am. J. Physiol., 1987, vol. 252, pp. E637–E642.
Miller, M., Kawabata, S., Wiltshire-Clement, M., Reventos, J., and Gordon, J.W., Increased vasopressin secretion and release in mice transgenic for the rat arginine vasopressin gene, Neuroendocrinology, 1993, vol. 57, pp. 621–625.
Montero, S., Mendoza, H., Valles, V., Lemus, M., Alvarez-Buylla, R., and de Alvarez-Buylla, E.R., Arginine-vasopressin mediates central and peripheral glucose regulation in response to carotid body receptor stimulation with Nacyanide, J. Appl. Physiol., 2006, vol. 100, pp. 1902–1909.
Nakamura, K., Aoyagi, T., Hiroyama, M., Kusakawa, S., Mizutani, R., Sanbe, A., Yamauchi, J., Kamohara, M., Momose, K., and Tanoue, A., Both V(1A) and V(1B) vasopressin receptors deficiency result in impaired glucose tolerance, Eur. J. Pharmacol., 2009, vol. 613, pp. 182–188.
Neumann, I.D. and Landgraf, R., Advances in vasopressin and oxytocin–from genes to behaviour to disease, Preface. Progr. Brain Res., 2008, vol. 170, vols. 11–13.
Nussey, S.S., Ang, V.T., Jenkins, J.S., Chowdrey, H.S., and Bisset, G.W., Brattleboro rat adrenal contains vasopressin, Nature, 1984, vol. 310, pp. 64–66.
Oba, Y. and Lone, N.A., Mortality benefit of vasopressor and inotropic agents in septic shock: A bayesian network meta-analysis of randomized controlled trials, J. Crit. Care, 2014, vol. 29, pp. 706–710.
Quintanar-Stephano, A., Organista-Esparza, A., Chavira-Ramirez, R., Kovacs, K., and Berczi, I., Effects of neurointermediate pituitary lobectomy and desmopressin on acute experimental autoimmune encephalomyelitis in lewis rats, Neuroimmunomodulation, 2012, vol. 19, pp. 148–157.
Ring, R.H., The central vasopressinergic system: Examining the opportunities for psychiatric drug development, Curr. Pharm. Design, 2005, vol. 11, pp. 205–225.
Roper, J.A., Craighead, M., O’Carroll, A.M., and Lolait, S.J., Attenuated stress response to acute restraint and forced swimming stress in arginine vasopressin 1b receptor subtype (Avpr1b) receptor knockout mice and wild-type mice treated with a novel Avpr1b receptor antagonist, J. Neuroendocrinol., 2010, vol. 22, pp. 1173–1180.
Schmale, H. and Richter, D., Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats, Nature, 1984, vol. 308, pp. 705–709.
Schmale, H., Ivell, R., Breindl, M., Darmer, D., and Richter, D., The mutant vasopressin gene from diabetes insipidus (Brattleboro) rats is transcribed but the message is not efficiently translated, EMBO J., 1984, vol. 3, pp. 3289–3293.
Serradeil-Le Gal, C., Bourrie, B., Raufaste, D., Carayon, P., Garcia, C., Maffrand, J.P., Le Fur, G., and Casellas, P., Effect of a new, potent, nonpeptide V1a vasopressin antagonist, SR 49059, on the binding and the mitogenic activity of vasopressin on Swiss 3t3 cells, Biochem. Pharmacol., 1994, vol. 47, pp. 633–641.
Serriere, V., Tran, D., Stelly, N., Claret, M., Alonso, G., Tordjmann, T., and Guillon, G., Vasopressin-induced morphological changes in polarized rat hepatocyte multiplets: Dual calcium-dependent effects, Cell Calcium, 2008, vol. 43, pp. 95–104.
Somova, L., Ivanova, E., Zaharieva, S., and Machuganska, A., Changes of adrenal vasopressin during hemorrhagic shock in rats with hereditary diabetes insipidus (Brattleboro strain), Acta Physiol. Pharmacol. Bulg., 1986, vol. 12, pp. 70–75.
Tahara, A., Tsukada, J., Tomura, Y., Yatsu, T., and Shibasaki, M., Vasopressin regulates rat mesangial cell growth by inducing autocrine secretion of vascular endothelial growth factor, J. Physiol. Sci., 2011, vol. 61, pp. 115–122.
Tamma, R., Sun, L., Cuscito, C., Lu, P., Corcelli, M., Li, J., Colaianni, G., Moonga, S.S., Di Benedetto, A., Grano, M., Colucci, S., Yuen, T., New, M.I., Zallone, A., and Zaidi, M., Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia, Proc. Natl. Acad. Sci. U.S.A., 2013, vol. 110, pp. 18644–18649.
Tanoue, A., Ito, S., Honda, K., Oshikawa, S., Kitagawa, Y., Koshimizu, T.A., Mori, T., and Tsujimoto, G., The vasopressin V1b receptor critically regulates hypothalamic-pituitary- adrenal axis activity under both stress and resting conditions, J. Clin. Invest., 2004, vol. 113, pp. 302–309.
Valtin, H., The discovery of the Brattleboro rat, recommended nomenclature, and the question of proper controls, Ann. N. Y. Acad. Sci., 1982, vol. 394, pp. 1–9.
Young, W.S., Kovacs, K., and Lolait, S.J., The diurnal rhythm in vasopressin v1a receptor expression in the suprachiasmatic nucleus is not dependent on vasopressin, Endocrinology, 1993, vol. 133, pp. 585–590.
Zelena, D., Vasopressin in health and disease with a focus on affective disorders, Central Nervous Syst. Agents Med. Chem, 2012, vol. 12, pp. 286–303.
Zhu, W., Tilley, D.G., Myers, V.D., Coleman, R.C., and Feldman, A.M., Arginine vasopressin enhances cell survival via a G protein-coupled receptor kinase 2/beta-arrestin1/extracellular-regulated kinase 1/2-dependent pathway in H9c2 cells, Mol. Pharmacol., 2013, vol. 84, pp. 227–235.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D. Zelena, 2016, published in Vavilovskii Zhurnal Genetiki i Selektsii, 2016, Vol. 20, No. 2, pp. 228–233.
Rights and permissions
About this article
Cite this article
Zelena, D. Comparison of natural and artificial vasopressin deficiency: Why is the latter lethal?. Russ J Genet Appl Res 7, 243–248 (2017). https://doi.org/10.1134/S2079059717030157
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079059717030157