Abstract
Frailty is the main geriatric syndrome, which is closely associated with age-related diseases and aging in general. Being considered the main pathogenetic mechanism of aging, low-grade chronic inflammation potentially contributes to increased degradation of the essential amino acid tryptophan through the kynurenine pathway. Active metabolites of the kynurenine pathway, when accumulated, implement their immunomodulatory, pro-inflammatory and cytotoxic properties, thereby supporting and enhancing the aging process. Over the past decade, data have been collected on the role of an unbalanced kynurenine pathway in the pathogenesis of frailty and age-related diseases. This review summarizes clinical and experimental data on the importance of kynurenine pathway analysis as a valuable tool for risk stratification and prognosis of frailty and age-related diseases.
Similar content being viewed by others
REFERENCES
Beard, J.R., Officer, A., de Carvalho, I.A., Sadana, R., Pot, A.M., Michel, J.P., Lloyd-Sherlock, P., Epping-Jordan, J.E., Peeters, G.M.E.E.G., Mahanani, W.R., Thiyagarajan, J.A., and Chatterji, S., The World report on ageing and health: A policy framework for healthy ageing, Lancet, 2016, vol. 387, no. 10033, pp. 2145–2154. PMID: 26520231; PMCID: PMC4848186.https://doi.org/10.1016/S0140-6736(15)00516-4
Fried, L.P., Tangen, C.M., Walston, J., Newman, A.B., Hirsch, C., Gottdiener, J., Seeman, T., Tracy, R., Kop, W.J., Burke, G., and McBurnie, M.A., Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype, J. Gerontol. A Biol. Sci. Med. Sci., 2001, vol. 56, no. 3, pp. M146–М156. PMID: 11253156.https://doi.org/10.1093/gerona/56.3.m146
Chen, X., Mao, G., and Leng, S.X., Frailty syndrome: An overview, Clin. Interv. Aging, 2014, vol. 9, pp. 433–441. PMID: 24672230; PMCID: PMC3964027.https://doi.org/10.2147/CIA.S45300
Ottenbacher, K.J., Graham, J.E., Al Snih, S., Raji, M., Samper-Ternent, R., Ostir, G.V., and Markides, K.S., Mexican Americans and frailty: Findings from the Hispanic established populations epidemiologic studies of the elderly, Am. J. Public Health, 2009, vol. 99, no. 4, pp. 673–679. https://doi.org/10.2105/AJPH.2008.143958
Suhre, K., Shin, S.Y., Petersen, A.K., et al., Human metabolic individuality in biomedical and pharmaceutical research, Nature, 2011, vol. 477, no. 7362, pp. 54–60. PMID: 21886157; PMCID: PMC3832838. https://doi.org/10.1038/nature10354
van der Greef, J., van Wietmarschen, H., van Ommen, B., and Verheij, E., Looking back into the future: 30 years of metabolomics at TNO, Mass. Spectrom. Rev., 2013, vol. 32, no. 5, pp. 399–415. PMID: 23630115.https://doi.org/10.1002/mas.21370
Stone, T.W. and Perkins, M.N., Quinolinic acid: A potent endogenous excitant at amino acid receptors in CNS, Eur. J. Pharmacol., 1981, vol. 72, no. 4, pp. 411–412. PMID: 6268428.https://doi.org/10.1016/0014-2999(81)90587-2
Moroni, F., Tryptophan metabolism and brain function: Focus on kynurenine and other indole metabolites, Eur. J. Pharmacol., 1999, vol. 375, nos. 1–3, pp. 87–100. PMID: 10443567. https://doi.org/10.1016/s0014-2999(99)00196-x
Ying, W., NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences, Antioxid. Redox Signal., 2008, vol. 10, no. 2, pp. 179–206. PMID: 18020963.https://doi.org/10.1089/ars.2007.1672
Jones, S.P., Franco, N.F., Varney, B., Sundaram, G., Brown, D.A., de Bie, J., Lim, C.K., Guillemin, G.J., and Brew, B.J., Expression of the kynurenine pathway in human peripheral blood mononuclear cells: Implications for inflammatory and neurodegenerative disease, PLoS One, 2015, vol. 10, no. 6, p. e0131389. PMID: 26114426; PMCID: PMC4482723.https://doi.org/10.1371/journal.pone.0131389
Majewski, M., Kozlowska, A., Thoene, M., Lepiarczyk, E., and Grzegorzewski, W.J., Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease, J. Physiol. Pharmacol., 2016, vol. 67, no. 1, pp. 3–19. PMID: 27010891.
Hardman, D., Aging: A theory based on free radical and radiation chemistry, J. Gerontol., 1956, vol. 11, no. 3, pp. 298–300. PMID: 13332224.https://doi.org/10.1093/geronj/11.3.298
Cervenka, I., Agudelo, L.Z., and Ruas, J.L., Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health, Science, 2017, vol. 357, no. 6349, p. eaaf9794. PMID: 28751584.https://doi.org/10.1126/science.aaf9794
Oxenkrug, G., Insulin resistance and dysregulation of tryptophankynurenine and kynurenine–nicotinamide adenine dinucleotide metabolic pathways, Mol. Neurobiol., 2013, vol. 48, no. 2, pp. 294–301. PMID: 23813101; PMCID: PMC3779535.https://doi.org/10.1007/s12035-013-8497-4
Vujkovic-Cvijin, I., Dunham, R.M., Iwai, S., et al., Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism, Sci. Transl. Med., 2013, vol. 5, no. 193, p. 193ra91. Erratum in: Sci Transl Med. 2017 Nov 8;9(415): PMID: 23843452; PMCID: PMC4094294.https://doi.org/10.1126/scitranslmed.3006438
Dehhaghi, M., Kazemi Shariat, P.H., and Guillemin, G.J., Microorganisms, tryptophan metabolism, and kynurenine pathway: A complex interconnected loop influencing human health status, Int. J. Tryptophan Res., 2019, vol. 12, p. 1178646919852996. https://doi.org/10.1177/1178646919852996
Heyes, M.P., Chen, C.Y., Major, E.O., and Saito, K., Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types, Biochem. J., 1997, vol. 326, pp. 351–356.
Moffett, J.R. and Namboodiri, M.A., Tryptophan and the immune response, Immunol. Cell Biol., 2003, vol. 81, no. 4, pp. 247–265. PMID: 12848846.https://doi.org/10.1046/j.1440-1711.2003.t01-1-01177.x
Heyes, M.P., Saito, K., Milstien, S., and Schiff, S.J., Quinolinic acid in tumors, hemorrhage and bacterial infections of the central nervous system in children. J. Neurol. Sci., 1995, vol. 133, nos. 1–2, pp. 112–118. PMID: 8583213.https://doi.org/10.1016/0022-510x(95)00164-w
Stone, T.W. and Addae, J.I., The pharmacological manipulation of glutamate receptors and neuroprotection, Eur. J. Pharmacol., 2002, vol. 447, pp. 285–296.
Cozzi, A., Carpenedo, R., and Moroni, F., Kynurenine hydroxylase inhibitors reduce ischemic brain damage: Studies with (m-nitrobenzoyl)-alanine (mNBA) and 3, 4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzen-esulfonamide (Ro 61–8048) in models of focal or global brain ischemia, J. Cereb. Blood Flow Metab., 1999, vol. 19, pp. 771–777.
Okuda, S., Nishiyama, N., Saito, H., and Katsuki, H., 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem., 1998, vol. 70, no. 1, pp. 299–307. PMID: 9422375.https://doi.org/10.1046/j.1471-4159.1998.70010299.x
Rapisarda, A., Pastorino, S., Massazza, S., Varesio, L., and Bosco, M., Antagonistic effect of picolinic acid and interferon-gamma on macrophage inflammatory protein-1a/b production, Cell Immunol., 2002, vol. 220, p. 7080.
Walston, J., McBurnie, M.A., Newman, A., et al., Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study, Arch. Intern. Med., 2002, vol. 162, no. 20, pp. 2333–2341.
Varadhan, R., Yao, W., Matteini, A., et al., Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults, J. Gerontol. A Biol. Sci. Med. Sci., 2014, vol. 69, no. 2, pp. 165–173.
Franceschi, C. and Campisi, J., Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases, J. Gerontol. A Biol. Sci. Med. Sci., 2014, vol. 69, suppl. 1, pp. S4–9.
Gonzalez-Freire, M., de Cabo, R., Studenski, S.A., and Ferrucci, L., The neuromuscular junction: Aging at the crossroad between nerves and muscle, Front. Aging Neurosci., 2014, vol. 6, p. 208.
Newman, A.B., Gottdiener, J.S., Mcburnie, M.A., Hirsch, C.H., Kop, W.J., Tracy, R., Walston, J.D., and Fried, L.P., Associations of subclinical cardiovascular disease with frailty, J. Gerontol. A Biol. Sci. Med. Sci., 2001, vol. 56, no. 3, pp. M158–М166. https://doi.org/10.1093/gerona/56.3.m158
Soysal, P., Stubbs, B., Lucato, P., Luchini, C., Solmi, M., Peluso, R., Sergi, G., Isik, A.T., Manzato, E., Maggi, S., Maggio, M., Prina, A.M., Cosco, T.D., Wu, Y.-T., and Veronese, N., Inflammation and frailty in the elderly: A systematic review and meta-analysis, Ageing Res. Rev., 2016, vol. 31, pp. 1–8. https://doi.org/10.1016/j.arr.2016.08.006
Hubbard, R.E., O’Mahony, M.S., Savva, G.M., Calver, B.L., and Woodhouse, K.W., Inflammation and frailty measures in older people, J. Cell. Mol. Med., 2009, vol. 13, no. 9B, pp. 3103–3109.https://doi.org/10.1111/j.1582-4934.2009.00733.x
Zhu, Y., Liu, Z., Wang, Y., Wang, Z., Shi, J., Xie, X., Jin, L., Chu, X., and Wang, X., C-reactive protein, frailty and overnight hospital admission in elderly individuals: A population-based study, Arch. Gerontol. Geriatr., 2016, vol. 64, pp. 1–5. https://doi.org/10.1016/j.archger.2015.08.009
Westbrook, R., Chung, T., Lovett, J., et al., Kynurenines link chronic inflammation to functional decline and physical frailty. JCI Insight, 2020, vol. 5, no. 16, p. e136091. PMID: 32814718; PMCID: PMC7455140.https://doi.org/10.1172/jci.insight.136091
Dugué, P.A., Hodge, A.M., Ulvik, A., et al., Association of markers of inflammation, the kynurenine pathway and B vitamins with age and mortality, and a signature of inflammaging, J. Gerontol. A Biol. Sci. Med. Sci., 2022, vol. 77, no. 4, pp. 826–836. PMID: 34117761. https://doi.org/10.1093/gerona/glab163
Walston, J., Fedarko, N., Yang, H., et al., The physical and biological characterization of a frail mouse model, J. Gerontol. A Biol. Sci. Med. Sci., 2008, vol. 63, no. 4, pp. 391–398.
Valdiglesias, V., Marcos-Pérez, D., Lorenzi, M., Onder, G., Gostner, J.M., Strasser, B., Fuchs, D., and Bonassi, S., Immunological alterations in frail older adults: A cross sectional study, Exp. Gerontol., 2018, vol. 112, pp. 119–126. PMID: 30240849.https://doi.org/10.1016/j.exger.2018.09.010
Marcos-Pérez, D., Sánchez-Flores, M., Maseda, A., Lorenzo-López, L., Millán-Calenti, J.C., Strasser, B., Gostner, J.M., Fuchs, D., Pásaro, E., Valdiglesias, V., and Laffon, B., Frailty status in older adults is related to alterations in indoleamine 2,3-dioxygenase 1 and guanosine triphosphate cyclohydrolase I enzymatic pathways. J. Am. Med. Dir. Assoc., 2017, vol. 18, no. 12, pp. 1049–1057. PMID: 28801236.https://doi.org/10.1016/j.jamda.2017.06.021
Fried, L.P., Tangen, C.M., Walston, J., Newman, A.B., Hirsch, C., Gottdiener, J., Seeman, T., Tracy, R., Kop, W.J., Burke, G., and McBurnie, M.A., Cardiovascular health study collaborative research group, frailty in older adults: Evidence for a phenotype, J. Gerontol. A Biol. Sci. Med. Sci., 2001, vol. 56, no. 3, pp. M146–М156. PMID: 11253156.https://doi.org/10.1093/gerona/56.3.m146
Rockwood, K. and Mitnitski, A., Frailty in relation to the accumulation of deficits, J. Gerontol. A Biol. Sci. Med. Sci., 2007, vol. 62, no. 7, pp. 722–727. PMID: 17634318.https://doi.org/10.1093/gerona/62.7.722
Theou, O., Brothers, T.D., Mitnitski, A., and Rockwood, K., Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality, J. Am. Geriatr. Soc., 2013, vol. 61, no. 9, pp. 1537–1551. PMID: 24028357.https://doi.org/10.1111/jgs.12420
Blodgett, J., Theou, O., Kirkland, S., Andreou, P., and Rockwood, K., Frailty in NHANES: Comparing the frailty index and phenotype, Arch. Gerontol. Geriatr., 2015, vol. 60, no. 3, pp. 464–470.https://doi.org/10.1016/j.archger.2015.01.016
Jang, I.Y., Park, J.H., Kim, J.H., Lee, S., Lee, E., Lee, J.Y., Park, S.J., Kim, D.A., Hamrick, M.W., and Kim, B.J., The association of circulating kynurenine, a tryptophan metabolite, with frailty in older adults, Aging (Albany NY), 2020, vol. 12, no. 21, pp. 22 253–22 265. PMID: 33188590.https://doi.org/10.18632/aging.104179
Westbrook, R., Chung, T., Lovett, J., et al., Kynurenines link chronic inflammation to functional decline and physical frailty, JCI Insight, 2020, vol. 5, no. 16, p. e136091. PMID: 32814718; PMCID: PMC7455140.https://doi.org/10.1172/jci.insight.136091
Dukes, A., Davis, C., El Refaey, M., Upadhyay, S., Mork, S., Arounleut, P., Johnson, M.H., Hill, W.D., Isales, C.M., and Hamrick, M.W., The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro, Nutrition, 2015, vol. 31, nos. 7–8, pp. 1018–1024. https://doi.org/10.1016/j.nut.2015.02.011
Mangge, H., Stelzer, I., Reininghaus, E.Z., Weghuber, D., Postolache, T.T., and Fuchs, D., Disturbed tryptophan metabolism in cardiovascular disease, Curr. Med. Chem., 2014, vol. 21, no. 17, p. 1931–1937. PMID: 24606499; PMCID: PMC4922792.https://doi.org/10.2174/0929867321666140304105526
Wokke, J.H., Jennekens, F.G., van der Oord, C.J., Veldman, H., Smit, L.M., and Leppink, G.J., Morphological changes in the human end plate with age, J. Neurol. Sci., 1990, vol. 95, no. 3, p. 291–310. PMID: 2358822.https://doi.org/10.1016/0022-510x(90)90076-y
Addi, T., Dou, L., and Burtey, S., Tryptophan-derived uremic toxins and thrombosis in chronic kidney disease, Toxins (Basel), 2018, vol. 10, no. 10, p. 412. PMID: 30322010; PMCID: PMC6215213.https://doi.org/10.3390/toxins10100412
Hsu, C.N. and Tain, Y.L., Developmental programming and reprogramming of hypertension and kidney disease: Impact of tryptophan metabolism, Int. J. Mol. Sci., 2020, vol. 21, no. 22, p. 8705. PMID: 33218054; PMCID: PMC7698939.https://doi.org/10.3390/ijms21228705
Rejdak, R., Junemann, A., Grieb, P., Thaler, S., Schuettauf, F., Choragiewicz, T., Zarnowski, T., Turski, W.A., and Zrenner, E., Kynurenic acid and kynurenine aminotransferases in retinal aging and neurodegeneration, Pharmacol. Rep., 2011, vol. 63, no. 6, pp. 1324–1334. PMID: 22358081.https://doi.org/10.1016/s1734-1140(11)70697-1
Al Saedi, A., Chow, S., Vogrin, S., Guillemin, G.J., and Duque, G., Association between tryptophan metabolites, physical performance, and frailty in older persons, Int. J. Tryptophan Res., 2022, vol. 15, p. 11786469211069951. PMID: 35125874; PMCID: PMC8808031.https://doi.org/10.1177/11786469211069951
Anaya, J.M., Bollag, W.B., Hamrick, M.W., and Isales, C.M., The role of tryptophan metabolites in musculoskeletal stem cell aging, Int. J. Mol. Sci., 2020, vol. 21, no. 18, p. 6670. https://doi.org/10.3390/ijms21186670
Darlington, L.G., Forrest, C.M., Mackay, G.M., Smith, R.A., Smith, A.J., Stoy, N., and Stone, T.W., On the biological importance of the 3-hydroxyanthranilic acid: Anthranilic acid ratio, Int. J. Tryptophan Res., 2010, vol. 3, pp. 51–59. https://doi.org/10.4137/ijtr.s4282
Isales, C., Ding, K., Bollag, W., McGee-Lawrence, M., Hill, W., Shi, X., Elsalanty, M., and Hamrick, M., Kynurenic acid a tryptophan metabolite induces bone loss in mice, Innov. Aging, 2018, vol. 2, pp. 100–101. https://doi.org/10.1093/geroni/igy023.377
Okuda, S., Nishiyama, N., Saito, H., and Katsuki, H., Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine, Proc. Natl. Acad. Sci. U.S.A., 1996, vol. 93, no. 22,pp. 12 553–12 558. https://doi.org/10.1073/pnas.93.22.12553
Aman, Y., Qiu, Y., Tao, J., and Fang, E., Therapeutic potential of boosting NAD+ in aging and age-related diseases, Transl. Med. Aging, 2018, vol. 2, pp. 30–37.
Ding, K., McGee-Lawrence, M.E., Kaiser, H., et al., Picolinic acid, a tryptophan oxidation product, does not impact bone mineral density but increases marrow adiposity, Exp. Gerontol., 2020, vol. 133, p. 110885.
Forrest, C.M., Mackay, G.M., Oxford, L., Stoy, N., Stone, T.W., and Darlington, L.G., Kynurenine pathway metabolism in patients with osteoporosis after 2 years of drug treatment, Clin. Exp. Pharmacol. Physiol., 2006, vol. 33, pp. 1078–1087.
Apalset, E.M., Gjesdal, C.G., Ueland, P.M., Midttun, Ø., Ulvik, A., Eide, G.E., Meyer, K., and Tell, G.S., Interferon (IFN)-γ-mediated inflammation and the kynurenine pathway in relation to bone mineral density: The Hordaland Health Study, Clin. Exp. Immunol., 2014, vol. 176, pp. 452–460.
Cason, C.A., Dolan, K.T., Sharma, G., Tao, M., Kulkarni, R., Helenowski, I.B., Doane, B.M., Avram, M.J., McDermott, M.M., Chang, E.B., Ozaki, C.K., and Ho, K.J., Plasma microbiome-modulated indole- and phenylderived metabolites associate with advanced atherosclerosis and postoperative outcomes, J. Vasc. Surg., 2018, vol. 68, no. 5, p. 1552–1562.e7. PMID: 29248242; PMCID: PMC5999545.https://doi.org/10.1016/j.jvs.2017.09.029
Wongpraparut, N., Pengchata, P., Piyophirapong, S., Panchavinnin, P., Pongakasira, R., Arechep, N., Kasetsinsombat, K., and Maneechotesuwan, K., Indoleamine 2,3 dioxygenase (IDO) level as a marker for significant coronary artery disease, BMC Cardiovasc. Disord., 2021, vol. 21, no. 1, p. 353. PMID: 34311709; PMCID: PMC8314527.https://doi.org/10.1186/s12872-021-02140-0
Bao, Y.S., Ji, Y., Zhao, S.L., Ma, L.L., Xie, R.J., and Na, S.P., Serum levels and activity of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA synthetase and their association with disease severity in patients with chronic kidney disease, Biomarkers, 2013, vol. 18, no. 5, p. 379–385. PMID: 23651343.https://doi.org/10.3109/1354750X.2013.790074
Rhee, E.P., Clish, C.B., Ghorbani, A., et al., A combined epidemiologic and metabolomic approach improves CKD prediction, J. Am. Soc. Nephrol., 2013, vol. 24, no. 8, p. 1330–1338. PMID: 23687356; PMCID: PMC3736702.https://doi.org/10.1681/ASN.2012101006
Silva, R.E., Baldim, J.L., Chagas-Paula, D.A., Soares, M.G., Lago, J.H.G., Goncalves, R.V., and Novaes, R.D., Predictive metabolomic signatures of endstage renal disease: A multivariate analysis of population-based data, Biochimie, 2018, vol. 152, pp. 14–30. PMID: 29913183.https://doi.org/10.1016/j.biochi.2018.06.009
Goek, O.N., Prehn, C., Sekula, P., Römisch-Margl, W., Döring, A., Gieger, C., Heier, M., Koenig, W., Wang-Sattler, R., Illig, T., Suhre, K., Adamski, J., Köttgen, A., and Meisinger, C., Metabolites associate with kidney function decline and incident chronic kidney disease in the general population, Nephrol. Dial. Transplant., 2013, vol. 28, no. 8, pp. 2131–2138. PMID: 23739151.https://doi.org/10.1093/ndt/gft217
Lee, H., Jang, H.B., Yoo, M.G., Park, S.I., and Lee, H.J., Amino acid metabolites associated with chronic kidney disease: An eight-year follow-up Korean epidemiology study, Biomedicines, 2020, vol. 8, no. 7, p. 222. PMID: 32708997; PMCID: PMC7399801.https://doi.org/10.3390/biomedicines8070222
Pertovaara, M., Raitala, A., Juonala, M., Lehtimäki, T., Huhtala, H., Oja, S.S., Jokinen, E., Viikari, J.S., Raitakari, O.T., and Hurme, M., Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: The cardiovascular risk in Young Finns Study, Clin. Exp. Immunol. 2007, vol. 148, no. 1, pp. 106–111. PMID: 17349013; PMCID: PMC1868844.https://doi.org/10.1111/j.1365-2249.2007.03325.x
Pedersen, E.R., Tuseth, N., Eussen, S.J., Ueland, P.M., Strand, E., Svingen, G.F., Midttun, Ø., Meyer, K., Mellgren, G., Ulvik, A., Nordrehaug, J.E., Nilsen, D.W., and Nygård, O., Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris, Arterioscler. Thromb. Vasc. Biol., 2015, vol. 35, no. 2, pp. 455–462. PMID: 25524770.https://doi.org/10.1161/ATVBAHA.114.304674
Crepaldi, G. and Allegri, G., Relationship between tryptophan metabolism and vitamin B6 and nicotinamid in aged subjects, Acta Vit. Enzymol., 1975, vol. 29, pp. 140–144.
Rogers, K.S. and Evangelista, S.J., 3-Hydroxykynurenine, 3-hydroxyanthranilic acid, and o-aminophenol inhibit leucine-stimulated insulin release from rat pancreatic islets, Proc. Soc. Exp. Biol. Med., 1985, vol. 178, no. 2, pp. 275–278. PMID: 3881773.https://doi.org/10.3181/00379727-178-42010
Price, J. and Brown, R., Quantitative studies on human urinary metabolites of tryptophan as affected by isoniazid and deoxypyridoxine, J. Clin. Invest., 1957, vol. 36, pp. 1600–1607.
Zuo, H., Ueland, P.M., Ulvik, A., Eussen, S.J., Vollset, S.E., Nygård, O., Midttun, Ø., Theofylaktopoulou, D., Meyer, K., and Tell, G.S., Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: The Hordaland Health Study. Am. J. Epidemiol., 2016, vol. 183, no. 4, pp. 249–258. PMID: 26823439; PMCID: PMC4753283.https://doi.org/10.1093/aje/kwv242
Liu, M., Wang, X., Wang, L., Ma, X., Gong, Z., Zhang, S., and Li, Y., Targeting the IDO1 pathway in cancer: From bench to bedside, J. Hematol. Oncol., 2018, vol. 11, no. 1, p. 100. PMID: 30068361; PMCID: PMC6090955.https://doi.org/10.1186/s13045-018-0644-y
Platten, M., Nollen, E.A.A., Röhrig, U.F., Fallarino, F., and Opitz, C.A., Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond, Nat. Rev. Drug Discov., 2019, vol. 18, no. 5, p. 379–401. PMID: 30760888.https://doi.org/10.1038/s41573-019-0016-5
Platten, M., von Knebel Doeberitz, N., Oezen, I., Wick, W., and Ochs, K., Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors, Front. Immunol., 2015, vol. 5, p. 673. PMID: 25628622; PMCID: PMC4290671.https://doi.org/10.3389/fimmu.2014.00673
Dukes, A., Davis, C., El Refaey, M., Upadhyay, S., Mork, S., Arounleut, P., Johnson, M.H., Hill, W.D., Isales, C.M., and Hamrick, M.W., The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro, Nutrition, 2015, vol. 31, pp. 1018–1024. https://doi.org/10.1016/j.nut.2015.02.011
El Refaey, M., Zhong, Q., Hill, W.D., Shi, X.M., Hamrick, M.W., Bailey, L., Johnson, M., Xu, J., Bollag, W.B., Chutkan, N., and Isales, C.M., Aromatic amino acid activation of signaling pathways in bone marrow mesenchymal stem cells depends on oxygen tension, PLoS One, 2014, vol. 9, p. e91108.
Ding, K.H., Cain, M., Davis, M., et al., Amino acids as signaling molecules modulating bone turnover, Bone, 2018, vol. 115, pp. 15–24.
Refaey, M.E., McGee-Lawrence, M.E., Fulzele, S., et al., Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss, J. Bone Miner. Res., 2017, vol. 32, pp. 2182–2193.
Kim, B.J., Hamrick, M.W., Yoo, H.J., Lee, S.H., Kim, S.J., Koh, J.M., and Isales, C.M., The detrimental effects of kynurenine, a tryptophan metabolite, on human bone metabolism, J. Clin. Endocrinol. Metab., 2019, vol. 104, no. 6, pp. 2334–2342. https://doi.org/10.1210/jc.2018-02481
Lovelace, M.D., Varney, B., Sundaram, G., Lennon, M.J., Lim, C.K., Jacobs, K., Guillemin, G.J., and Brew, B.J., Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases, Neuropharmacology, 2017, vol. 112, pp. 373–388.
Schwarcz, R. and Stone, T.W., The kynurenine pathway and the brain: Challenges, controversies and promises, Neuropharmacology, 2017, vol. 112, pp. 237–247.
Zadori, D., Nyiri, G., Szonyi, A., Szatmari, I., Fulop, F., Toldi, J., Freund, T.F., Vecsei, L., and Klivenyi, P., Neuroprotective effects of a novel kynurenic acid analogue in a transgenic mouse model of Huntington’s disease, J. Neural Transm. (Vienna), 2011, vol. 118, pp. 865–875.
Amaral, M., Levy, C., Heyes, D.J., Lafite, P., Outeiro, T.F., Giorgini, F., Leys, D., and Scrutton, N.S., Structural basis of kynurenine 3-monooxygenase inhibition, Nature, 2013, vol. 496, pp. 382–385.
Silva-Adaya, D., Perez-De La Cruz, V., Villeda-Hernandez, J., Carrillo-Mora, P., Gonzalez-Herrera, I.G., Garcia, E., Colin-Barenque, L., Pedraza-Chaverri, J., and Santamaria, A., Protective effect of L-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: Implications of modulating kynurenate as a protective strategy, Neurotoxicol. Teratol., 2011, vol. 33, no. 2, pp. 303–312.
Zádori, D., Nyiri, G., Szonyi, A., Szatmári, I., Fülöp, F., Toldi, J., Freund, T.F., Vécsei, L., and Klivényi, P., Neuroprotective effects of a novel kynurenic acid analogue in a transgenic mouse model of Huntington’s disease, J. Neural Transm. (Vienna), 2011, vol. 118, no. 6, pp. 865–875. PMID: 21194001.https://doi.org/10.1007/s00702-010-0573-6
Shi, H., Enriquez, A., Rapadas, M., et al., NAD deficiency, congenital malformations, and niacin supplementation, N. Engl. J. Med., 2017, vol. 377, no. 6, pp. 544–552. PMID: 28792876.https://doi.org/10.1056/NEJMoa1616361
Funding
Funding was provided under the Priority 2030 program.
Author information
Authors and Affiliations
Contributions
Developing the general concept of the review, literature search and analysis, writing the review text were performed by V.S. Pykhtina.
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants involved in the study.
CONFLICT OF INTEREST
The author of the work declares that she has no conflicts of interest.
Additional information
Translated by L. Solovyova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
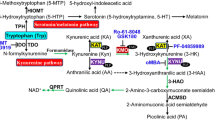
Abbreviations: 3-HAA, 3-hydroxyanthranilic acid; 3-HK, 3‑hydroxykynurenine; IL-1, interleukin; IL-6, interleukin; TNF-atumor necrosis factor alpha; IDO, indoleamine 2,3-dioxygenase; KYN, kynurenine; KYNA, kynurenic acid; KMO, kynurenine-3-monooxygenase; TDO, tryptophan 2,3-dioxygenase; TRP, tryptophan; QUIN, quinolinic acid; CNS, central nervous system; CKD, chronic kidney disease; IHD ischemic heart disease; CVD, Cardiovascular disease; NAD+, nicotinamide adenine dinucleotide; NADP nicotinamide adenine dinucleotide phosphate; PA, picolinic acid; NMDA, N-methyl-d-aspartate.
Rights and permissions
About this article
Cite this article
Pykhtina, V.S. The Role of Kynurenine Pathway Metabolites in the Development of Frailty in Older Adults. Adv Gerontol 13, 138–147 (2023). https://doi.org/10.1134/S2079057024600216
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079057024600216