Abstract
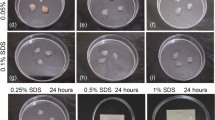
The main problem with decellularization of liver tissue as a tissue-specific matrix/scaffold in liver bioengineered structures is the need to maximize the preservation of the original three-dimensional structure of the tissue and the main components of its extracellular matrix (ECM) while removing cells and genetic material. The attempts to use the existing protocols for the decellularization of other tissues and organs have been unsuccessful. The aim of the work is to develop a method for creation of a tissue-specific microdispersed matrix from decellularized porcine liver (TMM DLp). The protocol for decellularization of porcine liver (Lp) fragments has been developed on the basis of the complex application of chemical (sodium dodecyl sulfate and Triton X100), biochemical (DNase I), and physical (supercritical CO2) methods for treatment of the initial tissue. As a result of the found optimal conditions for decellularization of Lp with subsequent cryomicronization of decellularized DLp, an injectable form of the microdispersed tissue-specific matrix was obtained, which represents decellularized porcine liver microparticles with the size of 100–200 μm with the residual amount of DNA no more than 10 ± 1.5 ng/mg (less than 1.0%), with the preservation of the microstructure and basic composition of the liver ECM. According to the in vitro assessment, biocompatible properties of tissue-specific matrix samples meet the criteria of biological safety for cytotoxicity and hemolytic activity.








Similar content being viewed by others
REFERENCES
Sevastianov, V.I., Technologies of tissue engineering and regenerative medicine, Vestn. Transplant. Iskusst. Organ., 2014, vol. 16, no. 3, pp. 93–108. https://doi.org/10.15825/1995-1191-2014-3-93-108
Pina, S., Ribeiro, V.P., Marques, C.F., Maia, F.R., Silva, T.H., Reis, R.L., and Oliveira, J.M., Scaffolding strategies for tissue engineering and regenerative medicine applications, Materials, 2019, vol. 12, no. 11, art. ID 1824. https://doi.org/10.3390/ma12111824
Rossi, E.A., Quintanilha, L.F., Nonaka, C.K.V., and Souza, B.S.F., Advances in hepatic tissue bioengineering with decellularized liver bioscaffold, Stem Cells Int., 2019, vol. 2019, art. ID 2693189. https://doi.org/10.1155/2019/2693189
Crapo, P.M., Gilbert, T.W., and Badylak, S.F., An overview of tissue and whole organ decellularization processes, Biomaterials, 2011, vol. 32, no. 12, pp. 3233–3243.
Tajima, K., Yagi, H., and Kitagawa, Y., Human-scale liver harvest and decellularization for preclinical research, in Decellularized Scaffolds and Organogenesis, Turksen, K., Ed., New York: Humana, 2018, vol. 1577, pp. 327–335.
Hassanein, W., Uluer, M.C., Langford, J., Woodall, J.D., Cimeno, A., Dhru, U., Werdesheim, A., Harrison, J., Rivera-Pratt, C., Klepfer, S., Khalifeh, A., Buckingham, B., Brazio, P.S., Parsell, D., Klassen, C., et al., Recellularization via the bile duct supports functional allogenic and xenogenic cell growth on a decellularized rat liver scaffold, Organogenesis, 2017, vol. 13, no. 1, pp. 16–27.
Wang, Y., Bao, J., Wu, Q., Zhou, Y., Li, Y., Wu, X., Shi, Y., Li, L., and Bu, H., Method for perfusion decellularization of porcine whole liver and kidney for use as a scaffold for clinical-scale bioengineering engrafts, Xenotransplantation, 2015, vol. 22, no. 1, pp. 48–61.
Ozlu, B., Ergin, M., Budak, S., Tunali, S., Yildirim, N., and Erisken, C., A bioartificial rat heart tissue: Perfusion decellularization and characterization, Int. J. Artif. Organs, 2019, vol. 42, no. 12, pp. 757–764.
Bölükbas, D.A., De Santis, M.M., Alsafadi, H.N., Doryab, A., and Wagner, D.E., The preparation of decellularized mouse lung matrix scaffolds for analysis of lung regenerative cell potential, in Mouse Cell Culture, Bertoncello, I., Ed., New York: Humana, 2019, vol. 1940, pp. 275–295.
Obata, T., Tsuchiya, T., Akita, S., Kawahara, T., Matsumoto, K., Miyazaki, T., Masumoto, H., Kobayashi, E., Niklason, L.E., and Nagayasu, T., Utilization of natural detergent potassium laurate for decellularization in lung bioengineering, Tissue Eng., Part C, 2019, vol. 25, no. 8, pp. 459–471.
Kuna, V.K., Kvarnström, N., Elebring, E., and Holgersson, S.S., Isolation and decellularization of a whole porcine pancreas, J. Visualized Exp., 2018, no. 140, art. ID 58302. https://doi.org/10.3791/58302
Nemec, E.A., Kirsanova, L.A., Basok, J.B., Shagidulin, M.J., Volkova, E.A., Metelsky, S.T., and Sevastianov, V.I., Technology features of decellularization of human liver fragments as tissue-specific fine-grained matrix for cell-engineering liver construction, Vestn. Transplant. Iskusst. Organ., 2017, vol. 19, no. 4, pp. 70–77.
Antons, J., Marascio, M.G., Aeberhard, P., Weissenberger, G., Hirt-Burri, N., Applegate, L.A., Bourban, P.E., and Pioletti, D.P., Decellularised tissues obtained by a CO2-philic detergent and supercritical CO2, Eur. Cell Mater., 2018, vol. 36, pp. 81–95. https://doi.org/10.22203/eCM.v036a15
Bernhardt, A., Wehrl, M., Paul, B., Hochmuth, T., Schumacher, M., Schütz, K., and Gelinsky, M., Improved sterilization of sensitive biomaterials with supercritical carbon dioxide at low temperature, PLoS One, 2015, vol. 10, no. 6, art. ID e0129205. https://doi.org/10.1371/journal.pone.0129205
GOST (State Standard) ISO 10993-5-2011: Medical Devices. Biological Evaluation of Medical Devices. Part 5. Tests for in vitro Cytotoxicity, 2014.
GOST (State Standard) ISO 10993-4-2011: Medical Devices. Biological Evaluation of Medical Devices. Part 4. Selection of Tests for Interactions with Blood, 2013.
Zhang, L., Guan, Z., Ye, J.-S., Yin, Y.-F., Stoltz, J.-F., and de Isla, N., Research progress in liver tissue engineering, Bio-Med. Mater. Eng., 2017, vol. 28, no. s1, pp. S113–S119. https://doi.org/10.3233/BME-171632
ACKNOWLEDGMENTS
This study was carried out with partial financial support from the Russian Foundation for Basic Research, grant no. 18-29-06012.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by L. Solovyova
Rights and permissions
About this article
Cite this article
Kirillova, A.D., Basok, Y.B., Lazhko, A.E. et al. Creating a Tissue-Specific Microdispersed Matrix from a Decellularized Porcine Liver. Inorg. Mater. Appl. Res. 12, 812–819 (2021). https://doi.org/10.1134/S2075113321030199
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113321030199