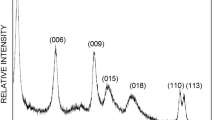
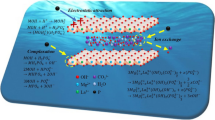
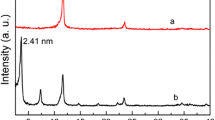
Abstract—Both traditional stone-like and novel microspheric ZnNiAl-layered double hydroxides (LDHs) were used as adsorbents for methyl orange removal from water. The stone-like ZnNiAl-LDH was synthesized by a coprecipitation method (c-LDH), and the microspheric ZnNiAl-LDH was prepared by a hydrothermal method (h-LDH). The special surface area of h-LDH (188.8 m2/g) is higher than that of c-LDH (71.0 m2/g), then h-LDH shows an excellent adsorption performance than c-LDH. The Al3+ content in microspheric ZnNiAl-LDH also has an impact on the adsorption capacity, which enhances with the Al3+ content increasing. The adsorption kinetics can be described with the pseudo-second-ordered model for both LDHs, and the adsorption isotherms of h-LDH can be fitted well by the Langmuir model, while the Freundlich model is better fit for c-LDH. The adsorption mechanisms for both LDHs are mainly attributed to ion exchange, electrostatic interaction and accompanied by surface complexation simultaneously.








Similar content being viewed by others
REFERENCES
Chen, Y.X., Jing, C., Zhang, X., et al., J. Colloid Interface Sci., 2019, vol. 548, p. 100–109.
Ding, D., Song, X., Wei, C.L., et al., Chemosphere, 2020, vol. 252, p. 126443.
Grover, A., Mohiuddin, I., Malik, A.K., et al., J. Hazard. Mater., 2022, vol, 424, p. 127454.
Mittal, J., J. Environ. Manage., 2021, vol. 295, p. 113017.
Lin, Y., Zeng, Z.K., Zhu, J.R., et al., Mater. Lett., 2015, vol. 156, p. 169–172.
Li, N., Chang, Z.D., Dang, H., et al., Colloids Surf., A, 2020, vol. 591, p. 124507.
Saghir, S., Fu, E.F. and Xiao, Z.G., Microporous Mesoporous Mater., 2020, vol. 297, p. 110010.
Zhu, Z.B., Ouyang, S.D., Li, P., et al., Appl. Clay Sci., 2020, vol. 188, p. 105500.
Lei, C.S., Pi, M., Kuang, P.Y., et al., J. Colloid Interface Sci., 2017, vol. 496, p. 158–166.
Pan, X.M., Zhang, M.M., Liu, H., et al., Appl. Surf. Sci., 2020, vol. 522, p. 146370.
Lyu, H.X., Hu, K., Fan, J.S., et al., Appl. Surf. Sci., 2020, vol. 500, p. 144037.
Mubarak, M., Islam, M.S., Yoon, D.Y., et al., Colloids Surf., A, 2021, vol. 618, p. 126446.
Cao, Y., Wang, Y.X., Zhang, X.X., et al., Mater. Lett., 2019, vol. 257, p. 126695.
Zhang, G.Z., Hu, L.M., Zhao, R.B., et al., J. Photochem. Photobiol., A, 2018, vol. 356, p. 633–641.
Costantino, U., Coletti, N. and Nocchetti, M., Langmuir, 1999, vol. 15, p. 4454–4460.
Yao, W., Yu, S.J., Wang, J., et al., Chem. Eng. J., 2017, vol. 307, p. 476–486.
Zheng, Y.M., Li, N. and Zhang, W.D., Colloids Surf., A, 2012, vol. 415, p. 195–201.
Jin, Z.X., Wang, X.X., Sun, Y.B., et al., Environ. Sci. Technol., 2015, vol. 49, p. 9168–9175.
Wang, J., Kang, D.J., Yu, X.L., et al., Chem. Eng. J., 2015, vol. 264, p. 506–513.
Sheng, G.D., Hu, J., Li, H., et al., Chemosphere, 2016, vol. 148, p. 227–232.
Chen, H., Zheng, Y.Q., Cheng, B., et al., J. Alloys Compd., 2018, vol. 735, p. 1041–1051.
Guan, T., Fang, L., Lu, Y., et al., Colloids Surf., A, 2017, vol. 529, p. 907–915.
Wang, L., Ge, S.S., Shao, Q., et al., Chin. J. Inorg. Chem., 2016, vol. 32, p. 1896–1904.
Hassani, K.E., Beakou, B.H., Kalnina, D., et al., Appl. Clay. Sci., 2017, vol. 140, p. 124–131.
Zhao, Y.G., Li, J., Zhang, S.W., et al., RSC Adv., 2013, vol. 3, p. 18952–18959.
Funding
This research is supported by the Industry-University-Research (IUR) Project of Ministry of Education (202002292014), the Scientific Research Fund of Henan Provincial Education Department (22A610010, 21A150032) and the Science and Technology Project of Henan Province (212102310511, 212102210590).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Cao, Y., Zhang, J., Zhou, P. et al. Effect of ZnNiAl-layered Double Hydroxide Morphology on Adsorption of Methyl Orange. Prot Met Phys Chem Surf 59, 133–142 (2023). https://doi.org/10.1134/S2070205123700314
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205123700314