Abstract
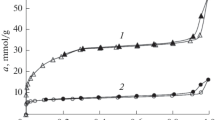
A Zn–BTB metal–organic framework structure was synthesized by the solvothermal method; it is characterized by micropore volume W0 = 0.67 cm3/g, effective radius of micropores х0 = 0.67 nm, and standard characteristic energy of benzene-vapor adsorption Е0 = 18.0 kJ/mol. The theory of volume filling of micropores was used to calculate the methane-adsorption equilibria on the synthesized Zn–BTB sample at temperatures over the range from 243 to 313 K and pressures up to 35 MPa; the differential molar isosteric heats of adsorption were evaluated. The maximum value of methane adsorption amounts to ~14.5 mmol/g at a pressure of 8 MPa and a temperature of 243 K.







Similar content being viewed by others
REFERENCES
Tsivadze, A.Yu., Aksyutin, O.E., Ishkov, A.G., Men’shchikov, I.E., Fomkin, A.A., Shkolin, A.V., Khozina, E.V., and Grachev, V.A., Usp. Khim., 2018, vol. 87, no. 10, p. 950.
Knyazeva, M.K., Solovtsova, O.V., Tsivadze, A.Yu., Fomkin, A.A., Shkolin, A.V., Men’shchikov, I.E., Pulin, A.L., Shiryaev, A.A., Vysotskii, V.V., and Kiselev, M.R., Russ. J. Inorg. Chem., 2019, vol. 64, no. 12, p. 1507.
Knyazeva, M.K., Tsivadze, A.Yu., Solovtsova, O.V., Fomkin, A.A., Pribylov, A.A., Shkolin, A.V., Pulin, A.L., and Men’shchikov, I.E., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 1, p. 9.
Knyazeva, M.K., Tsivadze, A.Yu., Fomkin, A.A., Shkolin, A.V., Solovtsova, O.V., Pribylov, A.A., Pulin, A.L., Yakovlev, V.Yu., and Men’shchikov, I.E., Prot. Met. Phys. Chem. Surf., 2020, vol. 56, no. 4, p. 682.
Zhao, D., Yuan, D., and Zhou, H.-C., Energy Environ. Sci., 2008, vol. 1, p. 222.
Alesaadi, S.J. and Sabzi, F., Int. J. Hydrogen Energy, 2015, vol. 40, no. 4, p. 1651.
Tsivadze, A.Yu., Aksyutin, O.E., Ishkov, A.G., Knyazeva, M.K., Solovtsova, O.V., Men’shchikov, I.E., Fomkin, A.A., Shkolin, A.V., Khozina, E.V., and Grachev, V.A., Usp. Khim., 2019, vol. 88, no. 9, p. 925.
Caskey, S.R., Wong-Foy, A.G., and Matzger, A.J., Inorg. Chem., 2008, vol. 47, p. 7751.
Chae, H.K., Siberio-Pérez, D.Y., Kim, J., Go, Y.-B., Eddaoudi, M., Matzger, A.J., O’Keeffe, M., and Yaghi, O.M., Nature, 2004, vol. 427, p. 523.
Zhang, Y.-B., Furukawa, H., Ko, N., Nie, W., Park, H.J., Okajima, S., Cordova, K.E., Deng, H., Kim, J., and Yaghi, O.M., J. Am. Chem. Soc., 2015, vol. 137, p. 2641.
Li, B., Wen, H.-M., Zhou, W., Xu, J.Q., and Chen, B., Chem, 2016, vol. 1, no. 4, p. 557.
Dubinin, M.M., Adsorbtsiya i poristost’ (Adsorption and Porosity), Moscow: Military Academy of Chemical Defense Named after Marshal of the USSR S.K. Timoshenko, 1972.
Men’shchikov, I.E., Fomkin, A.A., Tsivadze, A.Yu., et al., Prot. Met. Phys. Chem. Surf., 2015, vol. 51, p. 493.
Tsivadze, A.Yu., Aksyutin, O.E., Ishkov, A.G., et al., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, p. 24.
Chugaev, S.S., Fomkin, A.A., Men’shchikov, I.E., et al., Prot. Met. Phys. Chem. Surf., 2020, vol. 56, p. 897.
Kel’tsev, N.V., Osnovy adsorbtsionnoi tekhniki (Fundamentals of Adsorption Technique), Moscow: Khimiya, 1976.
Bakaev, V.A., Izv. Akad. Nauk SSSR, Ser. Khim., 1971, no. 2, p. 2648.
Fomkin, A.A., Adsorption, 2005, vol. 11, p. 425.
Funding
This work was carried out within the framework of a state assignment, theme no. 01201353185, using the equipment of the Center for Collective Use of the Frumkin Institute of Physical Chemistry and Electrochemistry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Khozina
Rights and permissions
About this article
Cite this article
Khyazeva, M.K., Fomkin, A.A., Shkolin, A.V. et al. Adsorption Properties of a Functional Porous Material Based on a Zn–BTB Metal–Organic Framework Structure. Prot Met Phys Chem Surf 58, 6–12 (2022). https://doi.org/10.1134/S2070205122010117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205122010117