Abstract
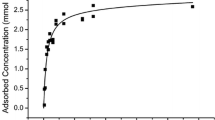
The adsorption of Ni(II), Zn(II), and Cu(II) ions by electrogenerated gibbsite (γ-modification aluminum hydroxide) has been studied. Electrically generated gibbsite was obtained by electrolysis of aqueous solutions using aluminum electrodes. We used gibbsite obtained during the first 5 min of electrolysis. Such an adsorbent has an amorphous state and a network structure and, as a consequence, has good adsorption capacity. The adsorption value for Ni(II) ions was 437.0 mg/g; for Zn(II), 362.5 mg/g; and for Cu(II), 148.8 mg/g. The obtained isotherms have a stepwise character, which is explained by the inhomogeneity of the adsorbing surface, on which there are groups of active centers that differ sharply from each other in their activity. The adsorption of toxic ions was studied using the Langmuir, Freundlich, and Dubinin–Radushkevich models. The values of the correlation coefficients indicate that the adsorption of Zn(II) Cu(II) ions is best described by the Langmuir model, and the adsorption of Ni(II) ions, by the Dubinin–Radushkevich model. On the basis of the Dubinin–Radushkevich adsorption model, the values of the free adsorption energy are determined, which indicate the physical nature of the interaction between the adsorptive and the adsorbent. The adsorption of toxic ions on the gibbsite surface occurs mainly due to dispersion interaction.





Similar content being viewed by others
REFERENCES
Abdel Salam, O.E., Reiad, N.A., and El-Shafei, M.M., J. Adv. Res., 2011, vol. 2, no. 4, pp. 297–303.
Sousa, F.W., Sousa, M.J., and Oliveira, I.R.N., J. Environ. Manage., 2009, vol. 90, no. 11, pp. 3340–3344.
Ji Fei, Li Chaolin, Xu Jialin, and Liu Peng, Colloids Surf., A, 2013, vol. 434, no. 5, pp. 88–94.
Wan Ngah, W.S., Teong, L.C., Toh, R.H., and Hanafiah, M.A.K.M., Chem. Eng. J., 2013, vol. 223, no. 1, pp. 231–238.
Qiu, W. and Ying Zheng, Y., Chem. Eng. J., 2009, vol. 145, no. 3, pp. 483–488.
Song, H., Song, H., Wan, X., Dai, M., Zhang, J., and Li, F., Fuel Process. Technol., 2013, vol. 116, pp. 52–62.
Keane, M., Colloids Surf., A, 1998, vol. 138, no. 1, pp. 11–20.
Oliveira, M.L.M., Miranda, A.A.L., Barbosa, C.M.B.M., Cavalcante, C.L., Jr., and Azevedo, D.C.S., Fuel, 2009, vol. 88, no. 10, pp. 1885–1892.
Filatova, E.G., J. Water Chem. Technol., 2016, vol. 38, no. 3, pp. 167–172.
Filatova, E.G. and Dudarev, V.I., Optimizatsiya elektrokoagulyatsionnoi ochistki stochnykh vod gal’vanicheskikh proizvodstv (Optimization for Electro-Coagulating Purifying Waste Waters of Galvanic Industry), Irkutsk: Irkutsk State Technical Univ, 2013.
Lur’e, Yu.Yu. and Rybnikova, A.I., Khimicheskii analiz proizvodstvennykh stochnykh vod (Chemical Analysis of Industrial Waste Waters), Moscow: Khimiya, 1974.
Marczenko, Z., Spectrophotometric Determination of the Elements, New York: John Wiley and Sons, 1976.
Babenkov, E.D., Vodu ochishchayut koagulyanty (Coagulants Purify Water), Moscow: Znanie, 1983.
Babenkov, E.D., Ochistka vody koagulyantami (Water Purification by Means of Coagulants), Moscow: Nauka, 1977.
Gel’fman, M.I., Kovalevich, O.V., and Yustratov, V.P., Kolloidnaya khimiya (Colloid Chemistry), St. Petersburg: Lan’, 2004.
Tsivadze, A.Yu., Rusanov, A.I., Fomkin A.A., et al., Fizicheskaya khimiya adsorbtsionnykh yavlenii (Physical Chemistry of Adsorption Phenomena), Moscow: Granitsa, 2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dudarev, V.I., Filatova, E.G. A Study of the Adsorption of Toxic Ions by Electrogenerated Gibbsite. Prot Met Phys Chem Surf 57, 283–288 (2021). https://doi.org/10.1134/S2070205121020052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205121020052