Abstract
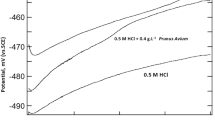
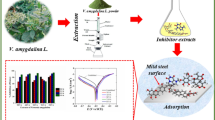
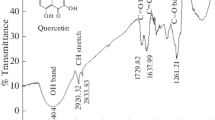
The thermodynamic, inhibitive and synergistic properties of Fragaria ananassa (strawberry) and Cucurbita pepo L (zucchini) leaf extracts on the corrosion of mild steel in Hydrochloric acid solutions were examined by potentiodynamic polarization curves measurements and electrochemical impedance spectroscopy (EIS) technique as well as optical photographic study. Fourier transform infrared spectroscopy (FTIR) and UV-Spectroscopy were applied to predict the possible functional groups and constituents of both leaf extracts. Potentiodynamic polarization curves proved that Fragaria ananassa and Cucurbita pepo L leaf extracts act as mixed type inhibitors for mild steel in 0.5 M HCl solutions. Impedance plots indicated that the corrosion process occurred under activation control. Thermodynamic parameters proved that the formation of the activated complex is an endothermic association instead of a dissociation step. The inhibitive efficiency of these leaf extracts relies on their physical adsorption on the metal surface rather than the complexation with Fe2+ ions. Cucurbita pepo L leaf extract is more efficient than Fragaria ananassa as a corrosion inhibitor for mild steel in 0.5 M HCl. The synergistic effect between both extracts may be due to co-adsorption between their molecules, which could be either competitive or co-operative adsorptions. The synergistic parameter value S was higher than unity indicating a cooperative synergistic effect.












Similar content being viewed by others
REFERENCES
Abdel-Gaber, A.M., Awad, R., Rahal, H.T., and Moussa, D., J. Bio- Tribo-Corros., 2019, vol. 5, p. 49.
El Sayed, M.Y., Abdel-Gaber, A.M., and Rahal, H.T., J. Failure Anal. Prev., 2019, vol. 19, pp. 1174–1180.
Alibakhshi, E., Ramezanzadeh, M., Bahlakeh, G., Ramezanzadeh, B., Mahdavian, M., and Motamedi, M., J. Mol. Liq., 2018, vol. 255, pp. 185–198.
Zhang, K., Yang, W., Yin, X., Chen, Y., Liu, Y., Le, J., and Xu, B., Carbohydr. Polym., 2018, vol. 181, pp. 191–199.
Aiad, I., Shaban, S.M., Moustafa, H.Y., and Hamed, A., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, pp. 135–147.
Ugryumov, O.V., Ivshin, Y.V., Fakhretdinov, P.S., Romanov, G.V., and Kaidrikov, R.A., Prot. Met. Phys. Chem. Surf., 2001, vol. 37, pp. 337–342.
Chadli, R., ELherri, A., Elmsellem, H., Elazzouzi, M., Merad, N., Aouniti, A., and Zarrouk, A., Prot. Met. Phys. Chem. Surf., 2017, vol. 53, pp. 928–936.
Rahal, H.T., Abdel-Gaber, A.M., and Younes, G.O., Chem. Eng. Commun., 2016, vol. 203, pp. 435–445.
Lai, C., Xie, B., Zou, L., Zheng, X., Ma, X., and Zhu, S., Results Phys., 2017, vol. 7, pp. 3434–3443.
Tsoeunyane, M.G., Makhatha, M.E., and Arotiba, O.A., Int. J. Corros., 2019, vol. 2019, article ID 7406409. https://doi.org/10.1155/2019/7406409
El-Haddad, M.A., Radwan, A.B., Sliem, M.H., Hassan, W.M., and Abdullah, A.M., Sci. Rep., 2019, vol. 9, p. 3695. https://doi.org/10.1038/s41598-019-40149-w
Hameed, R.A. and Abdallah, M., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, pp. 113–121.
Srivastava, M., Tiwari, P., Srivastava, S.K., Kumar, A., Ji, G., and Prakash, R., J. Mol. Liq., 2018, vol. 254, pp. 357–368.
Ehsani, A., Mahjani, M.G., Hosseini, M., Safari, R., Moshrefi, R., and Shiri, H.M., J. Colloid Interface Sci., 2017, vol. 490, pp. 444–451.
Al-Moghrabi, R.S., Abdel-Gaber, A.M., and Rahal, H.T., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, pp. 603–607.
Al-Moghrabi, R.S., Abdel-Gaber, A.M., and Rahal, H.T., Int. J. Ind. Chem., 2018, vol. 9, pp. 255–263.
Zheng, X., Gong, M., Li, Q., and Guo, L., Sci. Rep., 2018, vol. 8, p. 9140. https://doi.org/10.1038/s41598-018-27257-9
Akalezi, C.O., Onyedika, G.O., Chahul, H.F., and Oguzie, E.E., FUTO J. Ser.(FUTOJNLS), 2016, vol. 2, pp. 265–280.
Kalaiselvi, K., Chung, I.M., Kim, S.H., and Prabakaran, M., Anti-Corros. Methods Mater., 2018, vol. 65, pp. 408–416.
Boumhara, K., Harhar, H., Tabyaoui, M., Bellaouchou, A., Guenbour, A., and Zarrouk, A., J. Bio- Tribo-Corros., 2019, vol. 5, p. 8. https://doi.org/10.1007/s40735-018-0202-8
Tiwari, P., Srivastava, M., Mishra, R., Ji, G., and Prakash, R., J. Environ. Chem. Eng., 2018, vol. 6, pp. 4773–4783.
Bhuvaneswari, T.K., Vasantha, V.S., and Jeyaprabha, C., Silicon, 2018, vol. 10, pp. 1793–1807.
Mourya, P., Banerjee, S., and Singh, M.M., Corros. Sci., 2014, vol. 85, pp. 352–363.
Kumar, S., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, pp. 376–380.
Rajan, J.P., Shrivastava, R., and Mishra, R.K., Prot. Met. Phys. Chem. Surf., 2017, vol. 53, pp. 1161–1172.
Buyuksagis, A. and Dİlek, M., Prot. Met. Phys. Chem. Surf., 2018, vol. 55, pp. 1182–1194.
Haldhar, R., Prasad, D., and Bhardwaj, N., J. Adhes. Sci. Technol., 2019, vol. 33, pp. 1169–1183.
Ikeuba, A.I. and Okafor, P.C., Pigm. Resin Technol., 2019, vol. 48, pp. 57–64.
Mohammed, R.A. and AL-Mammar, D.E., Iraqi J. Sci., 2019, vol. 60, pp. 40–45.
Abdel-Gaber, A.M., Rahal, H.T., and Beqai, F.T., Int. J. Ind. Chem., 2020, vol. 11, pp. 123–132. https://doi.org/10.1007/s40090-020-00207-z
Abdel-Gaber, A.M., Abd-El-Nabey, B.A., Sidahmed, I.M., El-Zayady, A.M., and Saadawy, M., Corros. Sci., 2006, vol. 48, pp. 2765–2779.
Dehghani, A., Bahlakeh, G., and Ramezanzadeh, B., Bioelectrochemistry, 2019, vol. 130, p. 107339. https://doi.org/10.1016/j.bioelechem.2019.107339
Nikpour, S., Ramezanzadeh, M., Bahlakeh, G., Ramezanzadeh, B., and Mahdavian, B. M., Constr. Build. Mater., 2019, vol. 22, pp. 161–176.
Rosli, N.R., Yusuf, S.M., Sauki, A., and Razali, W.M.R.W., Key Eng. Mater., 2019, vol. 797, pp. 230–239.
Ogunleye, O.O., Eletta, O. A., Arinkoola, A.O., Agbede, O.O., Omodele, A.E., Morakinyo, A.F., and Osho, Y.A., IndianChem. Eng., 2019, vol. 2019, pp. 1–15.
Ogunleye, O.O., Eletta, O.A., Arinkoola, A.O., and Agbede, O.O., Asia-Pac. J. Chem. Eng., 2018, vol. 13, p. e2257. https://doi.org/10.1002/apj.2257
Liao, L.L., Mo, S., Luo, H.Q., and Li, N.B., J. Colloid Interface Sci., 2018, vol. 520, pp. 41–49.
Liao, L.L., Mo, S., Luo, H.Q., and Li, N.B., J. Colloid Interface Sci., 2017, vol. 499, pp.110–119.
Aiboudi, M., Yousfi, F., Fekkar, G., Bouyazza, L., Ramadani, M., El Azzouzi, M., and Abdelrahman, I., J. Mater. Environ. Sci., 2019, vol. 10, pp. 339–346.
Sanni, S.E., Fayomi, S.I., Emetere, M.E., and Tenebe, T.I., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, pp. 389–399.
Hedge, M. and Nayak, S.P., J. Mater. Environ. Sci., 2019, vol. 10, pp. 22–31.
Zhao, A., Sun, H., Chen, L., Huang, Y., Lu, X., Mu, B., Gao, H., Wang, S., and Singh, A., Int. J. Electrochem. Sci., 2019, vol. 14, pp. 6814–6825.
Dehghani, A., Bahlakeh, G., Ramezanzadeh, B., and Ramezanzadeh, M., J. Mol. Liq., 2019, vol. 279, pp. 603–624.
Umoren, S.A., Obot, I.B., and Gasem, Z.M., Ionics, 2015, vol. 21, p. 1171–1186.
Abdel-Gaber, A.M., Int. J. Appl. Chem., 2007, vol. 3, pp. 161–174.
El Din, A.S., Mohammed, R.A., and Haggag, H.H., Desalination, 1997, vol. 114, pp. 85–95.
Yaro, A.S., Khadom, A.A., and Idan, M.A., J. Mater. Environ. Sci., 2015, vol. 6, pp. 1101–1104.
Abdel-Gaber, A.M., Masoud, M.S., Khalil, E.A., and Shehata, E.E., Corros. Sci., 2009, vol. 51, pp. 3021–3024.
Rahal, H.T., Abdel-Gaber, A.M., Awad, R., and Abdel-Naby, B.A., Anti-Corros. Methods Mater., 2018, vol. 65, pp. 430–435.
Rahal, H.T., Abdel-Gaber, A.M., and Awad, R., Chem. Eng. Commun., 2017, vol. 204, pp. 348–355.
Tabesh, R.N., Abdel-Gaber, A.M., Hammud, H.H., and Al-Oweini, R., Z. Phys. Chem., 2019, vol. 233, pp. 1553–1569.
Lebrini, M., Robert, F., and Roos, C., Int. J. Electrochem. Sci., 2010, vol. 5, pp. 1698–1712.
Murakawa, T., Nagaura, S., and Hackerman, N., Corros. Sci., 1967, vol. 7, pp. 79–89.
Tavakoli, H., Shahrabi, T., and Hosseini, M.G., Mater. Chem. Phys., 2008, vol. 109, pp. 281–286.
Eduok, U.M., Umoren, S.A., and Udoh, A.P., Arabian J. Chem., 2012, vol. 5, pp. 325–337.
Loto, R.T. and Tobilola, O., J. King Saud Univ.,Eng. Sci., 2018, vol. 30, pp. 384–390.
Li, X., Deng, S., and Fu, H., Corros. Sci., 2009, vol. 51, pp. 1344–1355.
Chidiebere, M.A., Oguzie, E.E., Liu, L., Li, Y., and Wang, F., Ind. Eng. Chem. Res., 2014, vol. 53, pp. 7670–7767.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
El Khatib, L.W., Rahal, H.T. & Abdel-Gaber, A.M. Synergistic Effect between Fragaria ananassa and Cucurbita pepo L Leaf Extracts on Mild Steel Corrosion in Hydrochloric Acid Solutions. Prot Met Phys Chem Surf 56, 1096–1106 (2020). https://doi.org/10.1134/S2070205120050111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205120050111