Abstract
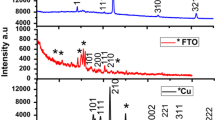
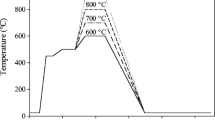
Pd–Pt thin films over titanium substrates were coated through electro-less deposition method as a function of pH values. It is concluded that highly acidic medium is helpful in achieving high amount of Pd–Pt coatings. The prepared thin films of different Pd–Pt ratios were characterized using the techniques of scanning electron microscopy and Energy dispersive X-ray spectroscopy. The catalytic efficiency was evaluated under static air conditions for hydrogen and oxygen recombination reaction. The developed catalysts have been found to be highly efficient for removal of hydrogen. Comparison of the hydrogen evaluation reaction, HER by Pd/Pt bimetallic catalysts with different percentages of Pd and Pt was measured and concluded that the hydrogen removal efficiency of catalyst having 35 wt % palladium and 65 wt % platinum (Pd35Pt65) prepared at pH value 1.2 was the best. The catalytic efficiency of all catalysts was in the range of 67% whereas the best sample was Pd35Pt65 deposited at a pH value of 1.2 and its efficiency was of the order of 86.7 ± 2%. Thus, it is concluded that these films may be used as sensors to monitor HER reactions and may be used at places where removal of atomic hydrogen is desired to avoid accidents.









Similar content being viewed by others
REFERENCES
Gheshlaghi, M.S., Seifzadeh, D., Shoghi, P. and Yangjeh, A.H., J. Alloys Compd., 2018, vol. 769, p. 149.
Uysal, M., Akbulut, H., Tokur, M., Algul, H., and Cetinkaya, T., J. Alloys Compd., 2016, vol. 654, p. 185.
Bahrami, F., Amini, R., and Taghvaei, A.H., J. Alloys Compd., 2017, vol. 714, p. 530.
Cabrera, A.L., Avila, J.I., and Lederman, D., Int. J. Hydrogen Energy, 2010, vol. 35, p. 10 613.
Agar, P., Mehta, B.R., Varandani, D., Arun, K.P., Kamruddin, M., and Tyagi, A.K., Sens. Actuators, B, 2010, vol. 150, p. 686.
De Robertis, E., Abrantes, L.M., and Motheo, A.J., Thin Solid Films, 2008, vol. 516, p. 6266.
Toshima, N. and Yonezawa, T., New J. Chem., 1998, vol. 22, p. 1179.
Sanjeev, G., Jerome, B., Studer, E., and Tkatschenko, I., Int. J. Hydrogen Energy, 2009, vol. 34, p. 5902-1.
IAEA Technical Report, Mitigation of Hydrogen Hazards in Severe Accidents in Nuclear Power Plants, IAEA-Tecdoc-1661, Vienna: International Atomic Energy Agency, 2011.
Coward, H.F. and Jones, G.W., Limits of Flammability of Gases and Vapors, Bulletin 503, US Bureau of Mines, Washington, DC: U.S. Government Printing Office, 1952.
Ahmed, B., Nicolas, M., and Alexandre, B., Nucl. Eng. Technol., 2015, vol. 47, p. 26.
Mabbott, S., Larmou, I.A., Vishnyakov, V., Xu, Y., Graham, D., and Goodacre, R., Analyst, 2012, vol. 137, no. 12, p. 27 918.
Magagnin, L., Maboudian, R., and Carraro, C., J. Phys. Chem. B, 2002, vol. 106, p. 401.
Ott, A., Bhargava, S.K., and O’Mullane, A.P., Surf. Sci., 2012, vol. 606, p. L59.
Porter, L.A., Choi, H.C., Ribbe, H.E., and Buriak, J.M., Nano Lett., 2002, vol. 2, p. 1067.
Lalik, E., Kosydar, R., Tokarz-Sobieraj, R., Witko, M., et.al., Appl. Catal., A, 2015, vol. 501, p. 27.
Ojani, R., Raoof, J.B., and Hasheminijed, E., Int. J. Hydrogen Energy, 2013, vol. 38, p. 92.
Norek, M., Stepniowski, M.J., Polanski, M., Zasada, D., Bojar, Z., and Bystrzycki, J., Int. J. Hydrogen Energy, 2011, vol. 36, p. 11 777.
Djokic, S.S., J. Electrochem. Soc., 1996, vol. 143, p. 1300.
Wang, D., Li, T., Liu, T., Huang, J., and You, T., Cryst. Growth Des., 2009, vol. 9, p. 4351e5.
Cabrera, A.L., Avila, J.I., and Lederma, D., Int. J. Hydrogen Energy, 2010, vol. 35, p. 10 613.
Wang, Z., Ida, T., Sakaue, H., Shingubara, S., and Takahagi, T., Electrochem. Solid-State Lett., 2003, vol. 6, no. 3, pp. C38–C41.
Kokkinidis, G., Papoutsis, A., Stoychev, D., and Milchev, A., J. Electroanal. Chem., 2000, vol. 486, p. 48.
Watanabe, H. and Honma, H., J. Electrochem. Soc., 1997, vol. 144, p. 471.
Chen, L. and Liu, Y., J. Colloid Interface Sci., 2011, vol. 364, p. 100.
Qu, L., Dai, L., and Osawa, E., J. Am. Ceram. Soc., 2006, vol. 128, p. 5523.
Kucernak, A.R.J., Fahy, K.F., and Sundaram, V.N., Catal. Today, 2016, vol. 262, p. 48.
Lalik, E., Drelinkiewicz, A., Kosydar, R., Rojek, W., et al., Int. J. Hydrogen Energy, 2015, vol. 40, p. 16 127.
Lali, F., Böttcher, G., Schöneich, P.-M., Haase, S., Hempel, S., and Lange, R., J. Eng. Res. Des., 2015, vol. 94, p. 365.
Ma1gorzata, N., Pniowski, W.J., Polanski, M., Zasada, D., Bojar, Z., and Bystrzycki, J., Int. J. Hydrogen Energy, 2011, vol. 36, p. 11 777.
Kelly, T.G., Hunt, S.T., Esposito, D.V., and Chen, J.G., Int. J. Hydrogen Energy, 2013, vol. 38, p. 5638.
Iwai, Y., Fusion Eng. Des., 2015, vols. 98–99, p. 1796.
Iwai, Y., Katsumi, S., and Yamanishi, T., J. Plasma Fusion Res. SER., 2010, vol. 9, p. 332.
Sanap, K.K., Varma, S., Dalavi, D., Patil, P.S., Waghmode, S.B., and Bharadwai, S.R., Int. J. Hydrogen Energy, 2011, vol. 36, p. 10 455.
Sanap, K.K., Varma, S., Waghmode, S.B., and Bharadwaj, S.R., Nucl. Eng. Des., 2015, vol. 294, p. 226.
Adriana, Z.-M., Marchelek, M., Diak, M., and Grabowska, E., Adv. Colloid Interface Sci., 2016, vol. 229, p. 80.
Cherevko, S., Geiger, S., Kasian, O., Kulyk, N., Grote, J.P., Savanc, A., et al., Catal. Today, 2016, vol. 262, p. 170.
París, R.S., L’Abbate, M.E., Liotta, L.F., et al., Catal. Today, 2016, vol. 275, p. 141.
Morfin, F., Sabroux, J.C., and Renouprez, A., Appl. Catal., B, 2004, vol. 47, p. 47.
Savadogo, O., Lee, K., Oishi, K., Mitsushima, S., Kamiya, N., and Ota, K.I., Electrochem. Commun., 2004, vol. 6, p. 105.
Chaokang, G., Brent, C.N., Fan, F.R.F., Bielawski, C.W., and Bard, A.J., ACS Catal., 2012, vol. 2, p. 746. https://doi.org/10.1021/cs3000107
Parka, J.W., Koh, B.R., and Suh, K.Y., Nucl. Eng. Des., 2011, vol. 241, p. 4280.
Shoaib Muhammad, M., PhM Thesis, Riphah International Univ., 2016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shoaib, M., Ul-Haq, N. & ul Haq, A. The Influence of pH on the Morphology and Electrochemical Behavior of Pd–Pt thin Films Prepared by Electro-less Deposition. Prot Met Phys Chem Surf 56, 785–792 (2020). https://doi.org/10.1134/S207020512004022X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207020512004022X