Abstract
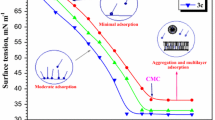
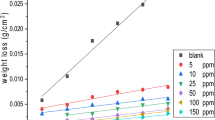
The corrosion and corrosion inhibition of Cu–Fe (20%) alloy are studied in 0.5 M H2SO4. Copper and iron samples are compared to the Cu–Fe alloy to understand the behavior of the Cu–Fe alloy in the corroding medium. Different techniques such as open circuit potential measurement, Tafel plots and electrochemical impedance spectroscopy (EIS) are used in this study. Dissolution of iron is found to be a controlling step in the corrosion behavior of the Cu-Fe alloy. Scanning electron microscope (SEM) and electron diffraction X-ray spectroscopy (EDX) charts confirm the above conclusion. The aggregation transition concentration (ATC) is determined and its value helps to interpret the CPBr inhibition behavior. Langmuir Adsorption isotherm is found to fit with the experimental data and relatively high negative value of the free energy of adsorption suggests strong adsorbability of the CPBr on the alloy surface.






Similar content being viewed by others
REFERENCES
Zuo, X., Qu, L., Zhao, C., An, B., Wang, E., Niu, R., Xin, Y., Lu, J., and Han, K., J. Alloys Compd., 2016, vol. 662, p. 355.
Gerengi, H., Schaefer, K., and Sahin, H.I., J. Ind. Eng. Chem., 2012, vol. 18, p. 2204.
Gerengi, H., Darowicki, K., Slepski, P., Bereket, G., and Ryl, J., J. Solid State Electrochem., 2010, vol. 14, p. 897.
Zhou, Q., Jiang, J., Zhong, Q., Wang, Y., Li, K., and Liu, H., J. Alloys Compd., 2013, vol. 563, p. 171.
Antonijevic, M.M. and Petrovic, M.B., Int. J. Electrochem. Sci., 2008, vol. 31, p. 1.
Nomoto, N., Chingping, T., Ohta, M., and Yamakawa, K., 62-Hitachi Cable Review, 1999, no. 18.
Superalloys II, Sims, C.T., Stoloff, N.S., and Hagel, W.C., Eds., New York: John Wiley and Sons, 1987, chap. 3, p. 61.
Gilbert, P.T., Mater. Perform., 1982, vol. 2, p. 47.
Mansfeld, F., Liu, G., Xiao, H., Tsai, C., and Little, B., Corros. Sci., 1994, vol. 36, p. 2063.
Equ, J., Guo, X, and Chen, Z., Mater. Chem. Phys., 2005, vol. 93, p. 388.
Talati, J.D. and Modi, R.M., Corros. Sci., 1979, vol. 19, p. 35.
Burzyniska, L., Maraszewska, A., and Zembura, Z., Corros. Sci., 1996, vol. 38, p. 337.
Mihit, M., El Issami, S., Bouklah, M., Bazzi, L., Hammouti, B., Ait Addi, E., Salghi, R., and Kertit, S., Appl. Surf. Sci., 2006, vol. 252, p. 2389.
Shayeb, H.A., Abd El Wahab, F.M., and Zein El Abedin, S., Br. Corros. J., 1999, vol. 34, p. 145.
Talati, J.D. and Modi, R.M., Br. Corros. J., 1977, vol. 12, p. 180.
Talati, J.D. and Modi, R.M., Corros. Prev. Control, 1977, vol. 24, p. 6.
Khamis, E., Bellucci, F., Latanision, R.M., and El Ashry, E.S., Corrosion, 1991, vol. 47, p. 677.
Yurt, A., Bereket, G., Kivrak, A., Balaban, A., and Erk, B., J. Appl. Electrochem., 2005, vol. 35, p. 1025.
Umoren, S.A., Ogbobe, O., Igwe, I.O., and Ebenso, E.E., Corros. Sci., 2008, vol. 50, p. 1998.
Okafor, P.C., Ikpi, M.E., Uwah, I.E., Ebenso, E.E., Ekpe, U.J., and Umoren, S.A., Corros. Sci., 2008, vol. 50, p. 2310.
Bentiss, F., Traisnel, M., and Lagrenee, M., Corros. Sci., 2000, vol. 42, p. 127.
Cruz, J., Martinez, R., Genesca, J., and Garcia-Ochoa, E., J. Electroanal. Chem., 2004, vol. 566, p. 111.
Abdennaby, A.M., Abdulhady, A.I., Abu-Oribi, S.T., and Saricimen, H., Corros. Sci., 1996, vol. 38, p. 1791.
Qu, Q., Jiang, S.A., Bai, W., and Li, L., Electrochim. Acta, 2007, vol. 52, p. 6811.
Awad, M.I. and Hazazi, O.A., Prot. Met. Phys. Chem. Surf., 2017, vol. 53, p. 236.
Kosari, A., Moayed, M.H., Davoodi, A., Parvizi, R., Momeni, M., and Eshghi, H., Corros. Sci., 2014, vol. 78, p. 138.
Larabi, L., Harek, Y., Benali, O., Ghalem, S., Larabi, L., Harek, Y., Benali, O., and Ghalem, S., Prog. Org. Coat., 2005, vol. 54, p. 256.
Fuchs-Godec, R. and Dolecek, V., Colloids Surf., A, 2004, vol. 244, p. 73.
Branzoi, V., Golgovici, F., and Branzoi, F., Mater. Chem. Phys., 2002, vol. 78, p. 122.
Khamis, A., Saleh, M.M., and Awad, M.I., Corros. Sci., 2013, vol. 66, p. 343.
Khamis, A., Saleh, M.M., Awad, M.I., and El-Anadouli, B.E., Corros. Sci., 2013, vol. 74, p. 83.
Osman, M.M., Mater. Chem. Phys., 2001, vol. 71, p. 12.
Abd El Rehim, S.S., Hassan, H.H., and Amin, M.A., Corros. Sci., 2004, vol. 46, p. 5.
Frignani, A., Grassi, V., Zanotto, F., and Zucchi, F., Corros. Sci., 2012, vol. 63, p. 29.
Zhou, Q., Jiang, J., Zhong, Q., Wang, Y., Li, K., and Liu, H., J. Alloys Compd., 2013, vol. 563, p. 171.
Riggs, O.L., Jr., in Corrosion Inhibitors, Nathan, C.C., Ed., Houston, TX: National Association of Corrosion Engineers, 1973.
Khamis, A., Saleh, M.M., Awad, M.I., and El-Anadouli, B.E., J. Adv. Res., 2014, vol. 5, p. 637.
Ma, H., Chen, S., Yin, B., Zhao, S., and Liu, X., Corros. Sci., 2003, vol. 45, p. 867.
Popova, A. and Christov, M., Corros. Sci., 2005, vol. 48, p. 3208.
Macdonald, D.D., Electrochim. Acta, 2006, vol. 51, p. 1376.
Yadav, M., Sinha, R.R., Sarkar, T.K., Bahadur, I., and Ebenso, E.E., J. Mol. Liq., 2015, vol. 212, p. 686.
Verma, C.B., Ebenso, E.E., Bahadur, I., Obot, I.B., and Quraishi, M.A., J. Mol. Liq., 2015, vol. 212, p. 209.
Hajjaji, N., Rico, I., Srhiri, A., Lattes, A., Soufiaoui, M., and Bachir, A.B., Corrosion, 1993, vol. 49, p. 326.
Elachouri, M., Hajji, M.S., Kertit, S., Essassi, E.M., Salem, M., and Coudert, R., Corros. Sci., 1995, vol. 37, p. 381.
Fuchs-Godec, R., Electrochim. Acta, 2009, vol. 54, p. 2171.
Asefi, D., Arami, M., and Mahmoodi, N.M., Corros. Sci., 2010, vol. 52, p. 794.
Fossa, M., Diplasb, S., and Gulbrandsenc, E., Electrochim. Acta, 2010, vol. 55, p. 4851.
Perche, T. and Anthore, R., Langmuir, 1996, vol. 12, p. 863.
Myer, D., Surfactants Science and Technology, New York: VCH Publ., 1988.
Atkins, P. and de Paula, J., in Physical Chemistry, W.H. Freeman, 2006.
Chebabe, D., Ait Chikh, Z., Hajjaji, N., Srhiri, A., and Zucchi, F., Corros. Sci., 2003, vol. 45, p. 309.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tammam, R.H., Saleh, M.M. Corrosion Inhibition of Copper-Iron Alloy in Acid Solution Using Cetylpyridinium Bromide as Cationic Surfactant. Prot Met Phys Chem Surf 55, 761–769 (2019). https://doi.org/10.1134/S2070205119040270
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119040270