Abstract
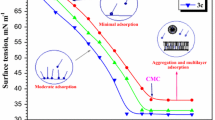
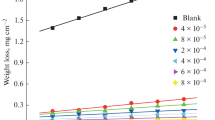
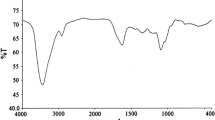
The quaternary ammonium salt-type tetrameric surfactant with hydrocarbon chain length of 12 was synthesized, and characterized using FT-IR and 1H NMR. The inhibition efficiencies for N80 steel in 20% hydrochloric acid were studied by weight loss, potentiodynamic polarization method, electrochemical impedance spectroscopy at 25°C. The results obtained from weigh loss measurements show that it has good inhibitive performance. Polarization data indicates that it acts as a mixed-type inhibitor. The results obtained from EIS exhibit the protective film formed by the adsorption of surfactant molecules on metal surface. The adsorption behavior of surfactants was found to follow the Langmuir adsorption isotherm.





Similar content being viewed by others
REFERENCES
Menger, F.M. and Littau, C.A., J. Am. Chem. Soc., 1991, vol. 113 pp. 1451–1452.
Menger, F.M. and Littau, C.A., J. Am. Chem. Soc., 1993, vol. 115, pp. 10083–10090.
Rosen, M.J. and Tracy, D.J., J. Surfactants Deterg., 1998, vol. 1, pp. 547–554.
Kabir, D., Siddiqui, U.S., Kumar, S., and Dar, A.A., Colloid Polym. Sci., 2006, vol. 284, pp. 807–812.
Zana, R., J. Colloid Interface Sci., 2002, vol. 248, pp. 203–220.
Ge, Y.S., Tai, S.X., Xu, Z.Q., Lai, L., Tian, F.F., Li, D.W., Jiang, F.L., Liu, Y., and Gao, Z.N., Langmuir, 2012, vol. 28, pp. 5913–5920.
Zhang, S.S., Yu, J., Wu, J.Z., Tong, W., Lei, Q.F., and Fang, W.J., J. Chem. Eng. Data, 2014, vol. 59, pp. 2891–2900.
Fan, Y.X., Hou, Y.B., Xiang, J.F., Yu, D.F., Wu, C.X., Tian, M.Z., Han, Y.C., and Wang, Y.L., Langmuir, 2011, vol. 27, pp. 10570–10579.
Koya, P.A., Wagay, T.A., and Ismail, K., J. Mol. Liq., 2016, vol. 219, pp. 505–512.
Yoshimura, T., Yoshida, H., Ohno, A., and Esumi, K., J. Colloid Interface Sci., 2003, vol. 267, pp. 167–172.
Laschewsky, A., Wattebled, L., Arotcarena, M., Habib-Jiwan, J.L., and Rakotoaly, R.H., Langmuir, 2005, vol. 21, pp. 7170–7179.
Marcelo, C.M., María, I.C., Javier, F.G., and Ricardo, J.G., Colloids Surf., A, 2005, vol. 262, pp. 1–7.
Yoshimura, T., Kimura, N., Onitsuka, E., Shosenji, H., and Esumi, K., J. Colloid Interface Sci., 2003, vol. 7, pp. 67–74.
Yang, F., Li, G., Qi, J., Zhang, S.M., and Liu, R., Appl. Surf. Sci., 2010, vol. 257, pp. 312–318.
Lagrenée, M., Mernari, B., Bouanis, M., Traisnel, M., and Bentiss, F., Corros. Sci., 2002, vol. 44, pp. 573–588.
Soliman, S.A., Metwally, M.S., Selim, S.R., Bedair, M.A., and Abbas, M.A., J. Ind. Eng. Chem., 2014, vol. 20, pp. 4311–4320.
Abd El-Lateef, H.M., Abo-Riya, M.A., and Tantawy, A.H., Corros. Sci., 2016, vol. 108, pp. 94–110.
Hegazy, M.A., Badawi, A.M., Abd El Rehim, S.S., and Kamel, W.M., Corros. Sci., 2013, vol. 69, pp. 110–122.
Al-Sabagh, A.M., Nasser, N.M., El-Azabawy, O.E., and El-Tabey, A.E., J. Mol. Liq., 2016, vol. 219, pp. 1078–1088.
Popova, A., Christov, M., and Vasilev, A., Corros. Sci., 2011, vol. 53, pp. 1770–1777.
Li, X.H., Deng, S.D., and Fu, H., Corros. Sci., 2011, vol. 53, pp. 302–309.
Free, M.L., Corros. Sci., 2002, vol. 44, pp. 2865–2870.
Free, M.L., Wang, W., and Ryu, D.Y., Corrosion, 2004, vol. 60, pp. 837–844.
Achouri, M.E., Infante, M.R., Izquierdo, F., et al., Corros. Sci., 2001, vol. 43, pp. 19–35.
Zana, R.J., J. Colloid Interface Sci., 2002, vol. 248, pp. 203–220.
Yoshimura, T., Yoshida, H., Ohno, A., et al., J. Colloid Interface Sci., 2003, vol. 267, pp. 167–172.
Tawfik, S.M., J. Mol. Liq., 2015, vol. 207, pp. 185–194.
Azzam, E.M.S., Hegazy, M.A., Kandil, N.G., Badawi, A.M., and Sami, R.M., Egypt. J. Pet., 2015, vol. 24, pp. 493–503.
Liu, X. and Zheng, Y.G., Corros. Eng., Sci. Technol., 2008, vol. 43, pp. 87–92.
Abd El-Maksoud, S.S. and Hassan, H.H., Mater. Corros., 2007, vol. 58, pp. 369–375.
Aiad, I., El-Sukkary, M.M., Soliman, E.A., El-Awady, M.Y., and Shaban, S.M., J. Ind. Eng. Chem., 2014, vol. 20, pp. 3524–3535.
Ferreira, E.S., Giancomelli, C., Giacomelli, F.C., and Spinelli, A., Mater. Chem. Phys., 2004, vol. 83, pp. 129–134.
Li, W.H., He, Q., Pei, C.L., and Hou, B.R., J. Appl. Electrochem., 2008, vol. 38, pp. 289–295.
Shaban, S.M., Abd-Elaal, A.A., and Tawfik, S.M., J. Mol. Liq., 2016, vol. 216, pp. 392–400.
Shaban, S.M., Aiad, I., El-Sukkary, M.M., Soliman, E.A., and El-Awady, M.Y., J. Mol. Liq., 2015, vol. 203, pp. 20–28.
Li, X.H., Deng, S.D., and Fu, H., Corros. Sci., 2012, vol. 55, pp. 280–288.
Pankaj, P., Arifa, S., and Ishtiaque, A., J. Surfactants Deterg., 2013, vol. 16, pp. 49–56.
Badr, G.E., Corros. Sci., 2009, vol. 51, pp. 2529–2536.
Abbasov, V.M., Abd El-Lateef, H.M., Aliyeva, L.I., Qasimov, E.E., Ismayilov, I.T., and Khalaf, M.M., Egypt. J. Pet., 2013, vol. 22, pp. 451–470.
Moretti, G., Guidi, F., and Grion, G., Corros. Sci., 2004, vol. 46, pp. 387–403.
Martinez, S. and Stern, I., Appl. Surf. Sci., 2002, vol. 199, pp. 83–89.
Szklarska-Smialowska, Z. and Mankowski, J., Corros. Sci., 1978, vol. 18, pp. 953–960.
Yurt, A., Ulutas, S., and Dal, H., Appl. Surf. Sci., 2006, vol. 253, pp. 919–925.
ACKNOWLEDGMENTS
The work was supported by the National Natural Science Foundation of China (grant no. 51074033)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Xie, Y., Li, W. et al. Synthesis and Inhibition Behavior of the Quaternary Ammonium Salt-type Tetrameric Surfactant for Corrosion of N80 Steel in HCl Medium. Prot Met Phys Chem Surf 55, 789–794 (2019). https://doi.org/10.1134/S2070205119040117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119040117