Abstract
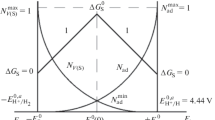
The concepts of the absolute surface potential (ASP) ES of the (hkl) facet of a metal crystal with ES = ΔGs/zF directly related to the Gibbs surface energy ΔGS and with an equilibrium between (auto) adsorption of its own atoms on this facet of Nad and negative charged surface vacancies (NCSV). On this basis, the ASP scale is a scale of adsorption potentials with point defects—the NCSV and adatoms, which determine their surface statistics as a result of the action of surface and electrostatic forces on these quasiparticles. This dualism is aimed at overcoming differences in the understanding of the surface potential in Helmgholz theory and Gibbs theory. The adsorption scale of the singular metal face has a special point—the potential of the zero charge (PZC) of the electrode \(E_{{\text{N}}}^{0}{\text{ = }}-\Delta G_{{\text{S}}}^{0}{{(hkl)} \mathord{\left/ {\vphantom {{(hkl)} {zF}}} \right. \kern-0em} {zF}}\) with a minimum of adsorption of atoms and NCSV. Point of absolute adsorption devides the scale of cathodic and anodic polarization (Fig. 4.1, Part 1) with predominant adsorption of NCSV or adatoms, reaching the maximum degree at the potential of the second special point \(E_{{\text{S}}}^{0}\) in each area of the ideal electrode polarization. Part 2 discusses the transition from the ASP to hydrogen scale using the ratio between the standard and absolute values of a hydrogen electrode adopted by the International Union of Physical and Applied Chemistry. Combining the ASP scale with the scale of the absolute potentials of electrode reactions made it possible to calculate the electrode potential of a chemisorption and electrocatalytic reaction of hydrogen evolution on various metals, as well as the potential for the formation of passivating oxide on metals (Ni, Cr), known as the “Flade potential.”


Similar content being viewed by others
REFERENCES
Andreev, Yu.Ya., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, no. 6, P. 991.
Bard, A.J., Inzelt, G., and Scholz, F., Electrochemical Dictionary, Berlin, Springer, 2012.
Bockris, J.O'M., Trans. Faraday Soc., 1947, vol. 43, p. 417.
Trassatti, S., J. Electroanal. Chem., 1972, vol. 39, p. 163.
Trasatti, S., Pure Appl. Chem., 1986, vol. 58, p. 955.
Trasatti, S. and Lust, E., The Potential of Zero Charge, in Modern Aspects of Electrochemistry no. 33, White, R.E., Bockris, J.O’M, and Conway, B.E., Eds., New York: Springer, 1999.
Andreev, Yu.Ya., Prot. Met. Phys. Chem. Surf., 2012, vol. 48, p. 290.
Vitos, L., Ruban, A.V., Skriver, H.L., and Kollar, J., Surf. Sci., 1998, vol. 441, p. 186.
Antropov, L.I., Teoreticheskaya elektrokhimiya (Theoretical Electrochemistry), Moscow: Vysshaya Shkola, 1975.
Roberts, M.W. and McKee, C.S., Chemistry of the Metal-Gas Interface, Oxford: Clarendon Press, 1978.
Frumkin, A.N., Izbrannye trudy. Elektrodnye protsessy (Selected Scientific Works. Electrode Processes), Moscow: Nauka, 1987.
Khaldeev, G.V., Strukturnaya korroziya metallov (Structural Metals Corrosion), Perm: Perm State Univ., 1994.
Horiuti, J. and Polanyi, U., Acta Physicochim. URSS, 1935, vol. 2, no. 4, p. 505.
Remy, H., Treatise on Inorganic Chemistry, Amsterdam, New York: Elsevier, 1956.
Andreev, Yu.Ya. and Bobkov T.V. Prot. Met. Phys. Chem. Surf., 2015, vol. 51, p. 730.
Andreev, Yu.Ya., Elektrokhimiya metallov i splavov (Electrochemistry of Metals and Alloys), Moscow: Vysshee Obrazovanie i Nauka, 2016.
Damaskin, B.B., Petrii, O.A., and Tcirlina, G.A. Elektrohimia (Electrochemistry), Moscow: KolosS, 2008.
Sato, N. and Okamoto, G., J. Electrochem. Soc., 1963, vol. 110, p. 605.
Haupt, S. and Strehblow, H.-H., J. Electroanal. Chem., 1987, vol. 228, p. 365.
Uhlig, H.H., Corrosion and Corrosion Control, New York: Wiley, 1962.
Kolotyrkin, Ya.M., Z. Elektrochem., 1958, vol. 62, p. 664.
Hoppe, H.-W. and Strehblow, H.-H., Surf. Interface Anal., 1989, vol. 14, p. 121.
Andreev, Ya.Ya., Safonov, I.A., and Doub, A.V., Prot. Met. Phys. Chem. Surf., 2010, vol. 46, p. 509.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Bannov
Rights and permissions
About this article
Cite this article
Andreev, Y.Y., Safonov, I.A. & Doub, A.V. Application of Scale of Absolute Surface Potentials to the Reactions of Chemisorption and Electrocatalysis on Metals. Part 2. Prot Met Phys Chem Surf 55, 1–8 (2019). https://doi.org/10.1134/S2070205119010039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119010039