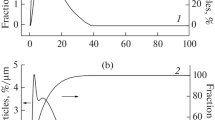
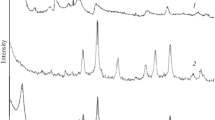
Abstract
A bentonite–magnetite composite is obtained by means of bentonite intercalation with iron oxide Fe3O4. Using the methods of electron microscopy, low-temperature nitrogen vapor adsorption–desorption, and XRD phase analysis, the composite morphology, structure, and texture have been investigated. It is shown that the synthesized bentonite–magnetite composite can be used as a sorbent for wastewater purification from organic pollutants.
Similar content being viewed by others
References
Handbook of Clay Science, Bergaya, F., Theng, B., and Lagaly, G., Eds., Amsterdam: Elsevier, 2006, vol. 1, p. 1224.
Gil, A., Korili, S.A., and Vicente, M.A., Catal. Rev., 2008, vol. 50, p. 153.
Divya, N., Bansal, A., and Jana, A.K., Bull. Chem. React. Eng. Catal., 2009, vol. 4, no. 2, p. 43.
Marzoog, A., Helal, M.I.D., and Khater, H.A., Int. J. Adv. Res., 2014, vol. 2, p. 1087.
Pastukhov, A.V., Davankov, V.A., Lubentsova, K.I., et al., Russ. J. Phys. Chem. A, 2013, vol. 87, no. 10, p. 1702–1708.
Bogachev, Yu.V., Gareev, K.G., Matyushkin, L.B., et al., Phys. Solid State, 2013, vol. 55, no. 12, pp. 2431–2435.
Arsalani, N., Fattahi, H., and Nazarpoor, M., eXPRESS Polym. Lett., 2010, vol. 4, p. 329.
Greg, S. and Sing, K., Adsorption, Surface Area and Porosity, New York Academic Press, 1982.
Kitaigorodskii, A.I., Rentgenostrukturnyi analiz melkokristallicheskikh i amorfnykh tel (X-ray Structural Analysis for Microcrystalline and Amorphous Solids), Moscow Gos. Izd. Tekhniko-Teoreticheskoi Literatury, 1952.
Vaganov, G.V., Yudin, V.E., Elokhovskii, V.Yu., et al., Russ. J. Appl. Chem., 2011, vol. 84, no. 8, pp. 1408–1413.
Peng Yuan, Mingde Fan, Dan Yang, et al., J. Hazard. Mater., 2009, vol. 166, p. 821.
Zhu, H.-Y., Fu, Y.-Q., Jiang, R., et al., Chem. Eng. J., 2011, vol. 173, p. 494.
Albornoz, C., Sileo, E.E., and Jacobo, S.E., Phys. B (Amsterdam, Neth.), 2004, vol. 354, p. 149.
Khashirova, S.Yu., Musaev, Yu.I., Mikitaev, A.K., Malkanduev, Yu.A., and Ligidov, M.Kh., Polym. Sci., Ser. A, 2009, vol. 51, nos. 9–10, pp. 377–382.
Silverstein, R.M., Bassler, G.C., and Morrill, T.C., Spectrometric Identification of Organic Compounds, New York John Wiley & Sons, 1991.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Alekseeva, A.N. Rodionova, N.A. Bagrovskaya, A.V. Agafonov, 2016, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2016, Vol. 52, No. 5, pp. 505–509.
Rights and permissions
About this article
Cite this article
Alekseeva, O.V., Rodionova, A.N., Bagrovskaya, N.A. et al. Synthesis, structure, and properties of a bentonite–magnetite composite. Prot Met Phys Chem Surf 52, 819–824 (2016). https://doi.org/10.1134/S2070205116050038
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205116050038