Abstract
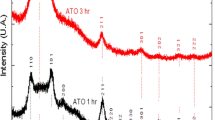
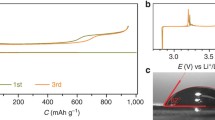
Electrocatalytic characteristics of a series of carbon materials (carbon blacks XC-72 and Super P and also multiwall nanotubes) and binary metallic nanosystems formed on carbon black XC-72 (PtRu/C and PdRu/C) are studied in the cathodic and anodic reactions of the positive electrode of a lithium–oxygen cell with nonaqueous electrolyte in the first discharge/charging cycles. It is found that a significant decrease in the cell charging overpotential is observed at a transition from carbon supports to binary systems. Overvoltage of the cathodic process also decreases when DMSO-based electrolyte is used in the case of binary systems. The obtained results are due to acceleration of oxygen reduction (cell discharge stage) and facilitation of lithium peroxide oxidation (cell charging stage) on the PtRu/C and PdRu/C systems.
Similar content being viewed by others
References
Yamamoto, O., in The Lithium Battery: Fundamentals, Imanishi, N., Luntz, A.C., and Bruce, P.G., Eds., New York: Springer-Verlag, 2014, p. 1.
Bruce, P.G., Freunberger, S., Hardwick, L., et al., Nat. Mater., 2012, vol. 11, no. 1, p. 19.
Chase, M.W. Jr., NIST-JANAF Thermochemical Tables: Monograph, Washington, D.C.: Am. Chem. Soc., 1998, 4th ed.
Zheng, J.P., Liang, R.Y., Hendrickson, M., et al., J. Electrochem. Soc., 2008, vol. 155, p. A432.
Imanishi, N. and Yamamoto, O., Mater. Today, 2014, vol. 17, p. 24.
Hummelshøj, J.S., Luntz, A.C., and Nørskov, J.K., J. Chem. Phys., 2013, vol. 138, p. 034703.
McCloskey, B.D., Scheffler, R., Speidel, A., et al., J. Am. Chem. Soc., 2011, vol. 133, p. 18038.
Lu, Y.-Ch., Gasteiger, H.A., Parent, M.C., et al., Electrochem. Solid-State Lett., 2010, vol. 13, p. A69.
Safari, M., Adams, B.D., and Nazar, L.F., J. Phys. Chem. Lett., 2014, vol. 5, p. 3486.
Dathar, G.K.P., Shelton, W.A., and Xu, Y., J. Phys. Chem. Lett., 2012, vol. 3, p. 891.
Greeley, J., Stephens, I.E.L., Bondarenko, A.S., et al., Nat. Chem., 2009, vol. 1, p. 552.
Lu, Y.-Ch., Gasteiger, H.A. and Shao-Horn, Y., J. Am. Chem. Soc., 2011, vol. 133, p. 19048.
Harding, J.R., Lu, Y.-Ch., Tsukada, Y., et al., Phys. Chem. Chem. Phys., 2012, vol. 14, p. 10540.
Sun, B., Munroe, P. and Wang, G., Sci. Rep., 2013, vol. 3, p. 2247.
Jian, Z., Liu, P., Li, F., et al., Angew. Chem. Int. Ed., 2014, vol. 53, p. 442.
Shen, Y., Sun, D., Yu, L., et al., Carbon, 2013, vol. 62, p. 288.
Albertus, P., Girishkumar, G., McCloskey, B., et al., J. Electrochem. Soc., 2011, vol. 158, p. A343.
Viswanathan, V., Thygesen, K.S., Hummelshoj, J.S., et al., J. Chem. Phys., 2011, vol. 135, p. 214704.
Tripachev, O.V., Korchagin, O.V., Bogdanovskaya, V.A., and Tarasevich, M.R., Russ. J. Electrochem., 2016, vol. 52, no. 5, p. 456.
Lu, Y.-Ch., Xu, Z., Gasteiger, H.A., et al., J. Am. Chem. Soc., 2010, vol. 132, p. 12170.
Yang, Y., Liu, W., Wang, Y., et al., Phys. Chem. Chem. Phys., 2014, vol. 16, p. 20618.
Avakov, T.B., Aliev, A.D., Bogdanovskaya, V.A., Ivanitskii, B.A., Kazanskii, L.P., Kapustin, A.V., Korchagin, O.V., Landgraf, I.K., Tarasevich, M.P., and Chalykh, A.E., Russ. J. Phys. Chem. A, 2015, vol. 89, no. 5, p. 887.
NIST X-ray Photoelectron Spectroscopy Database. http://srdata.nist.gov/xps/EnergyTypeValSrch.aspx.
Chakroune, N., Viau, G., Ammar, S., et al., Langmuir, 2005, vol. 21, p. 6788.
Tripachev, O.V., Maleeva, E.A., and Tarasevich, M.R., Russ. J. Electrochem., 2015, vol. 51, no. 2, p. 103.
Bogdanovskaya, V.A., Radina, M.V., Lozova, O.V., et al., Al’tern. Energ. Ekol., 2012, no. 2, p. 91.
Korchagin, O.V., Novikov, V.T., Rakov, E.G., Kuznetsov, V.V., and Tarasevich, M.R., Russ. J. Electrochem., 2010, vol. 46, no. 8, p. 882.
Soboleva, T., Zhao, X., Malek, K., et al., Appl. Mater. Interfaces, 2010, vol. 2, p. 375.
Ding, N., Chien, S.W., Hor, T.S.A., et al., J. Mater. Chem. A, 2014, vol. 2, p. 12433.
Yang, X.-H., He, P., and Xia, Y.-Y., Electrochem. Comm., 2009, vol. 11, p. 1127.
ASTM E1064–12: Standard test method for water in organic liquids by coulometric Karl Fischer titration. http://www.astm.org/Standards/E1064-RUS.htm.
Zhao, G., Niu, Y., Zhang, L., and Sun, K., J. Power Sources, 2014, vol. 270, p. 386.
Meini, S., Piana, M., Beyer, H., et al., J. Electrochem. Soc., 2012, vol. 159, p. A2135.
Gittleson, F.S., Sekol, R.C., Doubek, G., et al., Phys. Chem. Chem. Phys., 2014, vol. 16, p. 3230.
Xu, D., Wang, Z.-L., Xu, J.-J., et al., Chem. Comm., 2012, vol. 48, p. 6948.
Visco, S.J., Nimon, V., Petrov, A., et al., Lithium Air Batteries Based on Protected Lithium Electrodes in The Lithium Air Battery: Fundamentals, New York: Springer-Verlag, 2014, p. 179.
Johnson, L., Li, C., Liu, Z., et al., Nat. Chem., 2014, vol. 6, p. 1091.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Korchagin, M.R. Tarasevich, O.V. Tripachev, V.A. Bogdanovskaya, 2016, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2016, Vol. 52, No. 4, pp. 345–353.
Rights and permissions
About this article
Cite this article
Korchagin, O.V., Tarasevich, M.R., Tripachev, O.V. et al. Catalysis of oxygen reaction on positive electrode of a lithium–oxygen cell in the presence of metallic nanosystems. Prot Met Phys Chem Surf 52, 581–589 (2016). https://doi.org/10.1134/S2070205116020143
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205116020143