Abstract
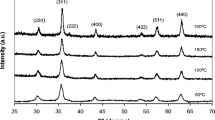
In the present work iron oxide nanoparticles have been synthesized by alkaline solvo thermal method using anhydrous ferric chloride, sodium hydroxide, polyethylene glycol and cetyl trimethyl ammonium bromide and characterized by X-ray diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR), Field Emission Scanning Electron Microscopy (FESEM), Energy-dispersive X-ray Spectroscopy (EDX) and Thermal Gravimetric Analysis (TGA). XRD indicated that the product is a mixture of different phases of iron oxide viz. gamma-Fe2O3 (maghemite, tetragonal), Fe2O3 (maghemite, cubic), Fe3O4 (magnetite, cubic) and ɛ-Fe2O3(epsilon iron oxide). FESEM studies indicated that size of the particles is observed in the range of about 19.8 nm to 48 nm. EDX spectral analysis reveals the presence of carbon, oxygen, iron in the synthesized nanoparticles. The FTIR spectra indicated absorption bands due to O-H stretching, C-O bending, N-H stretching and bending, C-H stretching and Fe-O stretching vibrations. TGA curve represented weight loss of around 3.0446 % in the sample at temperature of about 180°C due to the elimination of the water molecules absorbed by the nanoparticles from the atmosphere.
Similar content being viewed by others
References
Toyota, T., Ohguri, N., Maruyama, K., et al., Anal. Chem., 2012, vol. 84, p. 3952.
Chen, Y. and Chen, B.A., Chin J. Cancer., 2010, vol. 29, p. 125.
Behdadfar, B., Kermanpur, A., Sadeghi-Aliabadi, H., et al., Solid State Chem., 2012, vol. 187, p. 20.
Park, J.Y., Lee, Y.J., Khanna, P.K., et al., J. Molecular Catalysis A, 2010, vol. 323, p. 84.
Freund, B., Tromsdorf, U., Bruns, O., et al., ACS Nano, 2012, vol. 6, p. 7318.
Kulkarni, L., Group seminars “Synthesis and Characterization of Nanoparticles,” September, 2009.
Powder Diffraction File, Alphabetical Index Inorganic Phases, Pennsylvania: JCPDS Int. Centre For Diffraction Data, 1984.
Coates, J., Interpretation of Infrared Spectra, A Practical Approach, Encyclopedia of Analytical Chemistry, Meyers, A., Ed., Chichester: John Wiley & Sons Ltd, 2000, p. 10815.
Farmer, V.C., The Infrared Spectra of Minerals, London: Mineralogical Soc., 1974, p. 539.
Basavaraja, S., Balaji, D.S., Bedre, M.D., et al., Bull. Mater. Sci., 2011, vol.34, p. 1313.
Gadsden, J.A., Infrared Spectra of Minerals and Related Inorganic Compounds, England: Butterworths Publ., 1975.
Rahman, M.M., Khan, S.B, Jamal, A., et al., Iron Oxide Nanoparticles, ISBN: 978-953-307-913-4, 2011, p. 43, Intech Open access publisher.
Li, G.S. and Smith, R.L., Mater. Res. Bull., 2002, vol. 37, p. 949.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Mishra, D., Arora, R., Lahiri, S. et al. Synthesis and characterization of iron oxide nanoparticles by solvothermal method. Prot Met Phys Chem Surf 50, 628–631 (2014). https://doi.org/10.1134/S2070205114050128
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205114050128